Keywords
Maculopapular rash, renal transplant
Abstract
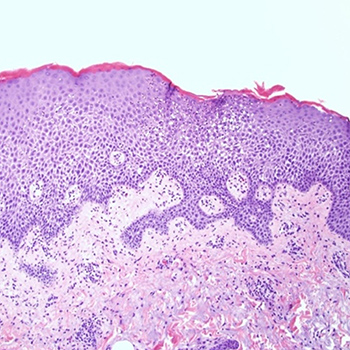
Sirolimus is an inhibitor of the mammalian target of rapamycin, which is used in kidney transplant immunosuppression. The clinical spectrum of cutaneous adverse events associated with sirolimus use varies, with maculopapular rash being an uncommon side effect very rarely reported in the literature. We present the case of a 78-year-old male renal transplant recipient who developed a diffuse maculopapular pruritic rash after starting sirolimus. This case report demonstrates that maculopapular rash is an uncommon sirolimus-related side effect that must be identified promptly so the medication can be discontinued and rash progression prevented.
References