Keywords
Gemfibrozil, anti-hyperlipidaemic, urinary side effects, polyuria
Abstract
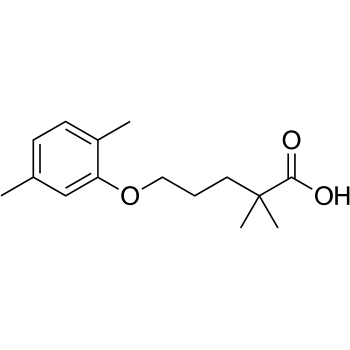
Gemfibrozil is a lipid-regulating agent used mainly to treat patients with hypertriglyceridaemia, especially those at risk for acute pancreatitis. Like any other pharmacological agent, gemfibrozil has known adverse effects, mainly gastrointestinal, such as cholelithiasis, gallstones, elevated transaminase, and other non-specific symptoms including dyspepsia, nausea and vomiting. Other reported adverse reactions are dizziness and vertigo, myopathy and rhabdomyolysis, angioedema, urticaria and rash. As far as we knew, gemfibrozil does not have urinary tract adverse reactions. In this report, we present a case of polyuria secondary to gemfibrozil with a score of 9 on the Naranjo scale, and a literature review.
References