Keywords
Trimebutine maleate, lichenoid drug eruption, lichen planus, cutaneous adverse reaction
Abstract
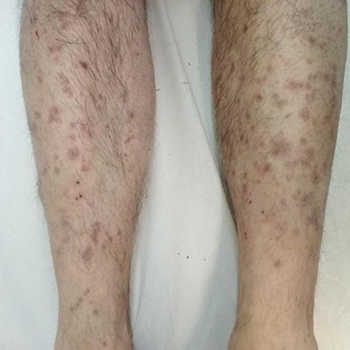
Trimebutine is a spasmolytic agent with antimuscarinic effects that is used for the treatment of irritable bowel syndrome (IBS) and lower gastrointestinal tract motility disorders. Lichenoid drug eruptions (LDE) to trimebutine maleate have not been previously reported. Here we present the case of a 50-year-old male patient who developed an extensive lichenoid eruption on his upper and lower extremities and trunk 4 weeks after starting treatment with trimebutine maleate 300 mg once daily for IBS. Two months after discontinuation of the drug and administration of topical treatment with emollients and corticosteroids, the LDE cleared completely with no recurrence. The diagnosis of LDE due to trimebutine was made, based upon the clinical features resembling lichen planus, the histological findings of interface dermatitis, the evidence of a temporal relationship between drug intake and the development of skin lesions, and resolution upon discontinuation of the drug. To the best of the authors’ knowledge, LDE following trimebutine maleate intake has not been previously reported. Management of trimebutine-induced LDE includes withdrawal of the causative agent and treatment with potent topical corticosteroids.
References
Views: 938
HTML downloads: 452
HTML downloads: 464
Published:
2020-11-24
Issue:
2020: Vol 7 No 12
(view)










