Keywords
Infection, viruses, cytomegalovirus, natalizumab, multiple sclerosis
Abstract
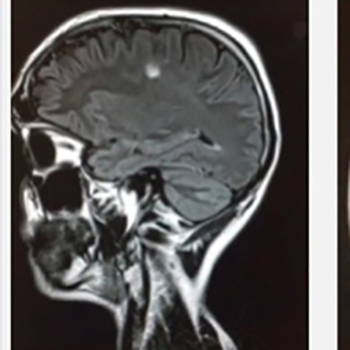
Natalizumab is indicated as monotherapy for the treatment of relapsing-remitting multiple sclerosis; it prevents outbreaks and delays the progression of physical disability. Here, we report the case of a 30-year-old patient with multiple sclerosis receiving natalizumab as monotherapy who subsequently developed self-limited cytomegalovirus disease.
Cytomegalovirus infection has been reported during treatment with natalizumab, and in this study, we use new techniques to analyze the possible association of cytomegalovirus infection with natalizumab.
References
Views: 1819
HTML downloads: 122
PDF downloads: 393
Published:
2019-02-01
Issue:
Vol 6 No 2
(view)










