Abstract
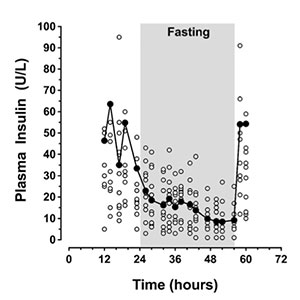
Liraglutide, a glucagon-like peptide-1 (GLP-1) analog, is increasingly used in obese patients with type 2 diabetes mellitus (T2DM) in doses of up to 3.0 mg/day because of its attractive pharmacological profile. It is currently not known how to proceed with this medication during fasting for surgery. Discontinuation is likely to result in hyperglycaemia, while continuation might lead to hypoglycaemia, but, in view of its mode of action, continuation of GLP-1 analogs is likely to be safe. However, as evidence-based guidelines on GLP-1 management during perioperative fasting are not available, the safety of either policy needs to be confirmed on an individual basis. We therefore decided to perform a preoperative assessment of the glucose response to fasting during continuation of GLP-1 before giving a recommendation in individual cases. So far, 12 severely obese T2DM patients scheduled for bariatric surgery have been evaluated preoperatively by measuring glucose and insulin levels during a 32-hour fast with continuation of liraglutide. Hypoglycaemia was not observed. This suggests that liraglutide in doses of up to 3.0 mg can be safely continued during surgery without risking hypoglycaemia.
References