Keywords
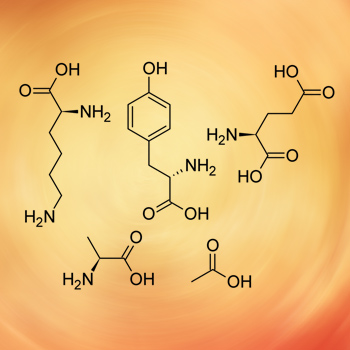
Glatiramer acetate, drug associated immune thrombocytopenic purpura, thrombocytopenia.
Abstract
We present a case of glatiramer acetate-associated refractory immune thrombocytopenic purpura (ITP) in a female patient with multiple sclerosis. A search of MEDLINE/PubMed did not find any connection between glatiramer acetate and thrombocytopenia, specifically ITP. The autoimmune reaction was resistant to conservative ITP treatment, and was eventually managed only by splenectomy. To the best of our knowledge, this is the first report of glatiramer acetate-associated ITP. Physicians should be aware of this condition, and consider performing routine blood counts at the beginning of glatiramer acetate treatment.
References