ABSTRACT
The maze procedure for atrial fibrillation carries risks, including pleural effusion. We report a case of a 54-year-old woman with right-sided pleural effusion post maze surgery, presenting with dyspnoea. Despite treatment, complications arose, including atrial flutter. Prompt recognition and multidisciplinary management led to a favourable outcome. This case underscores the importance of vigilance for rare post-operative complications and highlights the need for collaborative care in optimising patient outcomes following cardiac surgeries. Further research is warranted to refine management strategies for such occurrences.
KEYWORDS
Haemothorax, MAZE procedure, pleural effusion
LEARNING POINTS
- Healthcare providers should remain vigilant for rare complications, for example right-sided haemothorax, following cardiac surgeries such as the maze procedure to initiate timely management and ensure favourable outcomes.
- The post-maze procedure, atrial flutter or macroreentrant atrial tachycardia may resist standard medical treatment, emphasising the importance of considering catheter ablation as a therapeutic option to improve patient outcomes.
- Empowering patients with knowledge about potential post-procedure complications and associated symptoms facilitates early reporting, enabling prompt intervention by healthcare providers and leading to improved treatment outcomes.
INTRODUCTION
The maze procedure, a surgical intervention aimed at treating atrial fibrillation, involves creating a pattern of scar tissue within the heart’s upper chambers to disrupt abnormal electrical signals responsible for the arrhythmia[1,2]. While generally considered safe, the procedure carries inherent risks and potential complications including bleeding, infection and the development of pleural effusion. Pleural effusion is more commonly observed as left-sided or bilateral effusion. Instances of isolated right-sided pleural effusion following the maze procedure are rare but can occur. In the presented case, the patient developed a massive right-sided pleural effusion, highlighting the importance of recognising and managing this uncommon complication.
CASE DESCRIPTION
A middle-aged female presented to the emergency department with worsening shortness of breath one month following cardiovascular surgery. Her medical history was significant for non-occlusive coronary artery disease, non-ischaemic cardiomyopathy with a reduced ejection fraction of 25–30% heart failure with reduced ejection fraction (HFrEF) status post automated implantable defibrillator (AICD) placement. She also had chronic atrial fibrillation with intermittent rapid ventricular response (RVR) status post left atrial appendage ligation, severe mitral valve regurgitation status post mitral valve repair, type 2 diabetes mellitus and amiodarone-induced hyperthyroidism. She underwent an uneventful elective maze one month previously. She had been experiencing progressively worsening shortness of breath and orthopnoea since the procedure, limiting her activity and causing symptoms at rest.
On presentation, she was tachycardic with a heart rate of 122 beats per minute and was hypoxic in room air, requiring 2 l of supplemental nasal cannula oxygen, but otherwise had an unremarkable vital examination. The physical examination revealed a patient in mild respiratory distress, diminished breath sounds in bilateral lung auscultation with the right greater than the left, and bilateral pitting pedal oedema.
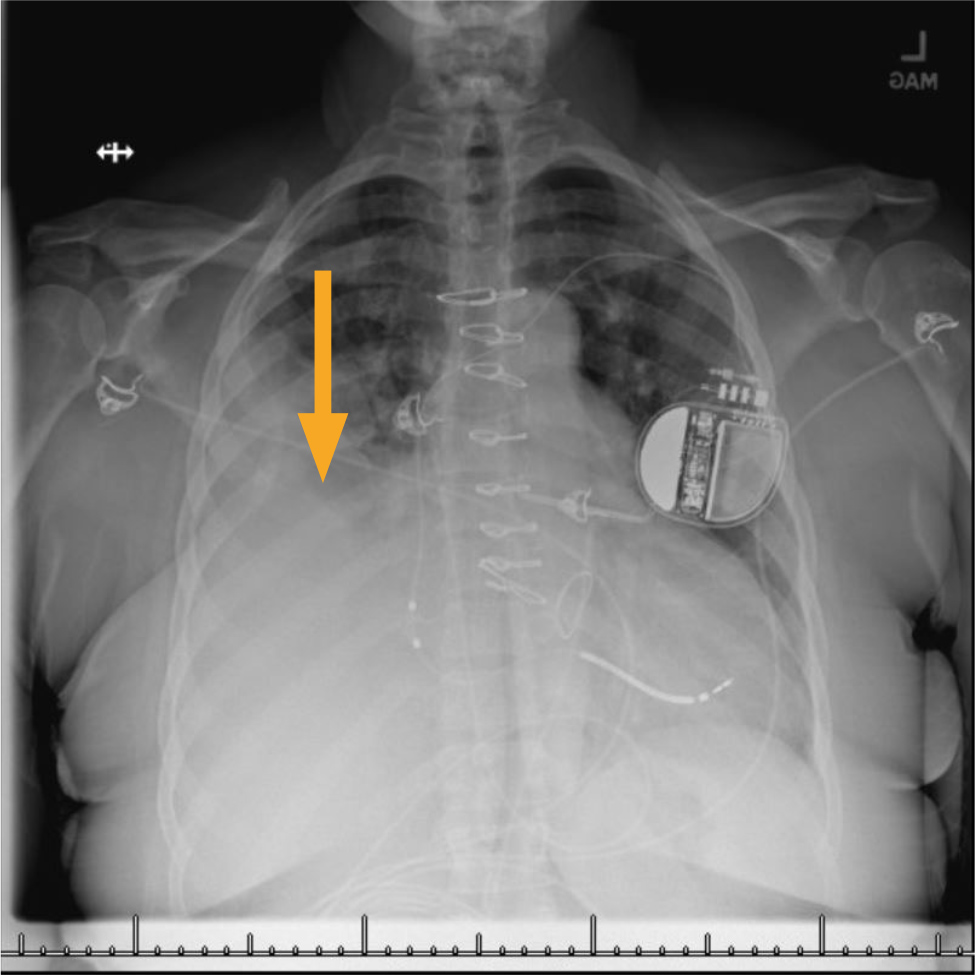
Blood workup included mildly elevated troponin of 0.1 ng/ml which subsequently trended down, brain natriuretic peptide of 278 pg/ml, blood urea nitrogen of 40 ml/dl, serum creatinine of 1.2 mg/dl (baseline 0.6 mg/dl), haemoglobin of 10.5 g/dl (baseline) and a platelet count of 287,000 cells/Ul. An electrocardiogram (ECG) was negative for ST elevation myocardial infarction but showed 2:1 atypical atrial flutter (AF) with RVR. Chest X-ray showed right-sided pleural effusion (Fig. 1). Computed tomography angiography (CTA) of the chest was negative for pulmonary embolism but revealed massive right-sided pleural effusion. She underwent right-sided thoracentesis, with the removal of 1,400 ml of blood-tinged fluid, the analysis of which was consistent with exudate, along with negative culture with a few atypical cells in cytology.
The initial working diagnosis was acute on chronic systolic heart failure, acute pulmonary embolism, acute coronary syndrome (ACS) and post-operative complications. CTA of the chest ruled out acute pulmonary embolism; negative ECG and static troponin were against the diagnosis of ACS. Though the presence of massive right-sided effusion was initially supporting the diagnosis of acute or chronic systolic heart failure, the blood-tinged fluid and exudative nature of the pleural fluid led to the diagnosis of right haemothorax secondary to complication from the recent maze procedure. It is hypothesised that this has led to the AF with RVR, and the presenting symptoms as well.
Following right-side thoracentesis, AF with RVR was initially treated with oral amiodarone, and rivaroxaban was resumed 48 hours later. She continued to be in AF with RVR, with hypotension precluding the use of rate control medication. Given the persistent AF with RVR and impending haemodynamic instability, she was planned for transoesophageal echocardiogram (TEE) with cardioversion. Following TEE, she was given midazolam for cardioversion shortly after which she became bradycardic and had pulseless electrical activity cardiac arrest. Return of spontaneous circulation was achieved following two minutes of cardiopulmonary resuscitation (CPR) and flumazenil administration. She remained neurologically intact. She continued to be in AF with RVR, with failed rate and rhythm control medication. Hence, she underwent atrioventricular (AV) node ablation and upgrading to biventricular ICD.
The rest of the hospital course was uneventful. She was initiated on guidelines-directed medical therapy for HFrEF and was subsequently discharged. On two weeks follow-up with her primary care physician, she denied dyspnoea on exertion, or orthopnoea. Follow-up with the cardiologist two months later, she continued to report improved symptoms with no further adverse events.
DISCUSSION
Atrial fibrillation is commonly associated with mitral valve disease due to the underlying pathophysiology, leading to left atrial enlargement with a prevalence of 30–50% in patients presenting for mitral valve surgery[2,3]. Thus, it is not uncommon to perform concomitant mitral valve repair with the maze procedure for atrial fibrillation as an open surgical procedure, as in our patient. Although generally considered safe, like any surgical intervention, the maze procedure carries potential risks and complications such as bleeding, infection and the development of pleural effusion[4,5].
Pleural effusion can occur following any cardiac procedure due to various factors, including inflammation and direct injury to the pleura or associated vessels when it manifests as haemothorax. While the exact frequency of pleural effusion post-maze procedure remains poorly documented, it commonly manifests as left-sided or bilateral effusion. Instances of isolated right-sided pleural effusion such as in our patient following the maze procedure are rare[5]. Though the exact mechanism of the right haemothorax in our patient is unclear, we suspect it could be a combination of inflammation induced by surgical trauma, lymphatic disruption and haemorrhage during the procedure as described as the mechanism of early non-specific pleural effusion, which is manifested within 30 days post cardiac surgery. The treatment approach for depends on the underlying cause. It will resolve spontaneously if it is small. But in symptomatic, massive pleural effusion and haemothorax, therapeutic thoracentesis with chest tube placement may be necessary[6].
In a meta-analysis to assess the recurrence of atrial fibrillation after concomitant maze and mitral valve surgery, this was found to be 67.1% at 12 months[7]. In another study, it is mentioned that they can also develop macroreentrant atrial tachycardia or AF, as in our patient[8,9]. These are often resistant to medical therapy and require catheter ablation. Our patient failed medical management; hence cardioversion was attempted which also failed, so catheter ablation was done as a last resort with a successful outcome.
CONCLUSION
In conclusion, the case manifests two rare complications post maze surgery including right-sided haemothorax and AF. Yet, the patient had a good clinical outcome, underscoring the importance of prompt recognition and management of complications in the post-operative period to optimise patient outcomes. Collaborative multidisciplinary care involving cardiologists, cardiothoracic surgeons and critical care specialists is crucial in ensuring timely diagnosis and appropriate management of such complications. Further research is warranted to better understand the incidence, risk factors and optimal management strategies for these rare complications in the context of cardiac surgeries.