ABSTRACT
Calcium plays a crucial role in the heart’s electrical conduction system and facilitating the contraction of cardiac muscles. Hypocalcemia can result in electrocardiogram findings such as a prolonged QTC interval and eventually torsade de pointes, which in severe cases can progress to cardiac arrest. In cases of B-cell lymphoma, hypocalcemia may arise from various factors. Tumor infiltration can disrupt calcium homeostasis by affecting the parathyroid glands or bone tissue. Acidosis in the context of B-cell lymphoma can cause significant cardiovascular adverse effects. It will reduce peripheral vascular resistance and cardiac muscle contractility, promote dysrhythmias, and disturb oxygen uptake in the lungs. These combined effects markedly compromise cardiac function, increasing the likelihood of cardiac arrest. These mechanisms necessitate comprehensive management strategies in B-cell lymphoma patients. In this case report we present a case of cardiac arrest in a 59-year-old female woman with hypocalcemia and lactic acidosis secondary to B-cell lymphoma.
KEYWORDS
Cardiac arrest, B-cell lymphoma, hypocalcemia
LEARNING POINTS
- Lactic acidosis in B-cell lymphoma can be multifactorial. Contributing factors include inability of liver lactate clearance, tumor cell metabolism or impaired oxygenation.
- Patients with B-cell lymphoma may have hypocalcemia secondary to tumor lysis syndrome, paraneoplastic syndrome, or secondary to treatment.
- These reversible causes should always be considered in cardiac arrest in cancer patients.
INTRODUCTION
Calcium is vital for the proper functioning of the heart’s electrical conduction system and the contraction of cardiac muscles[1]. When there is a deficiency of calcium, known as hypocalcemia, it can lead to the prolongation of the QT interval on an electrocardiogram (ECG)[2]. This prolonged QT interval can predispose individuals to a particular type of ventricular tachycardia called torsade de pointes, which can progress to cardiac arrest[3]. Another contributing factor to cardiac arrest in patients with B-cell lymphoma is lactic acidosis. Like hypocalcemia, lactic acidosis is also multifactorial. There are multiple contributing factors like liver involvement that may affect its ability to clear lactic acid, sepsis, high tumor cells turnover or impaired oxygen delivery. In this article we report cardiac arrest in a 59-year-old female diagnosed with B-cell lymphoma, in the setting of hypocalcemia and lactic acidosis.
CASE DESCRIPTION
A 59-year-old female with lupus, immune thrombocytopenic purpura (ITP), splenectomy, essential hypertension, anemia and jugular vein thrombosis not on anticoagulation presented to the emergency department (ED) with a 3-4 days history of fatigue, hematemesis, diarrhea and dyspnea. Upon arrival she was hypotensive, tachycardic, tachypneic and confused. Remarkable laboratory results are reported in Table 1.
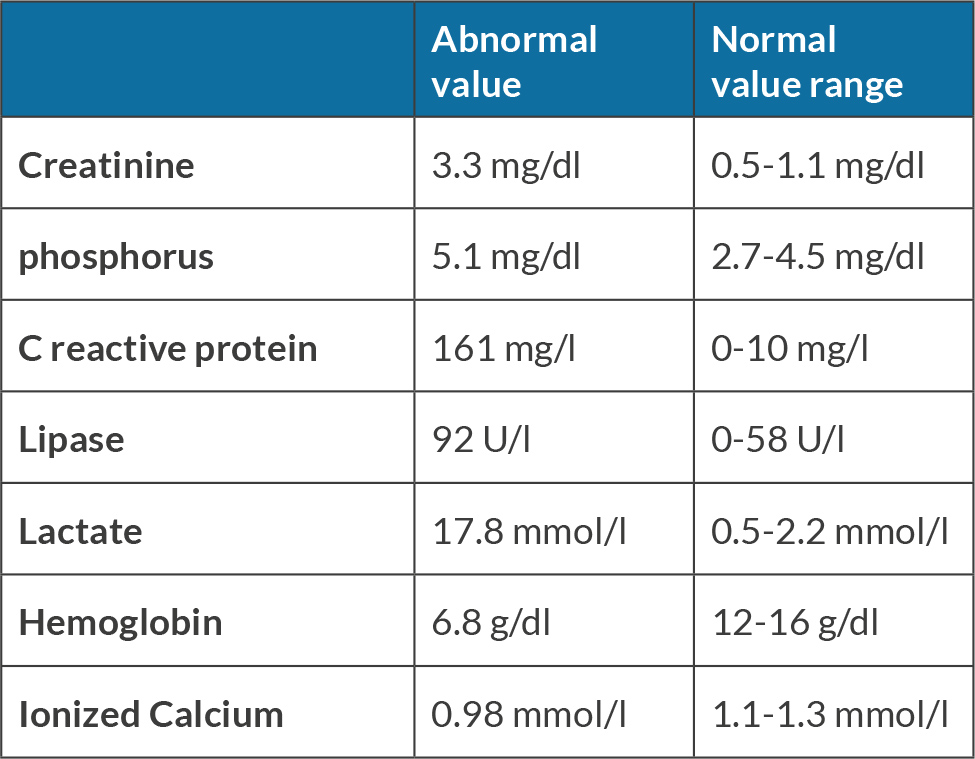
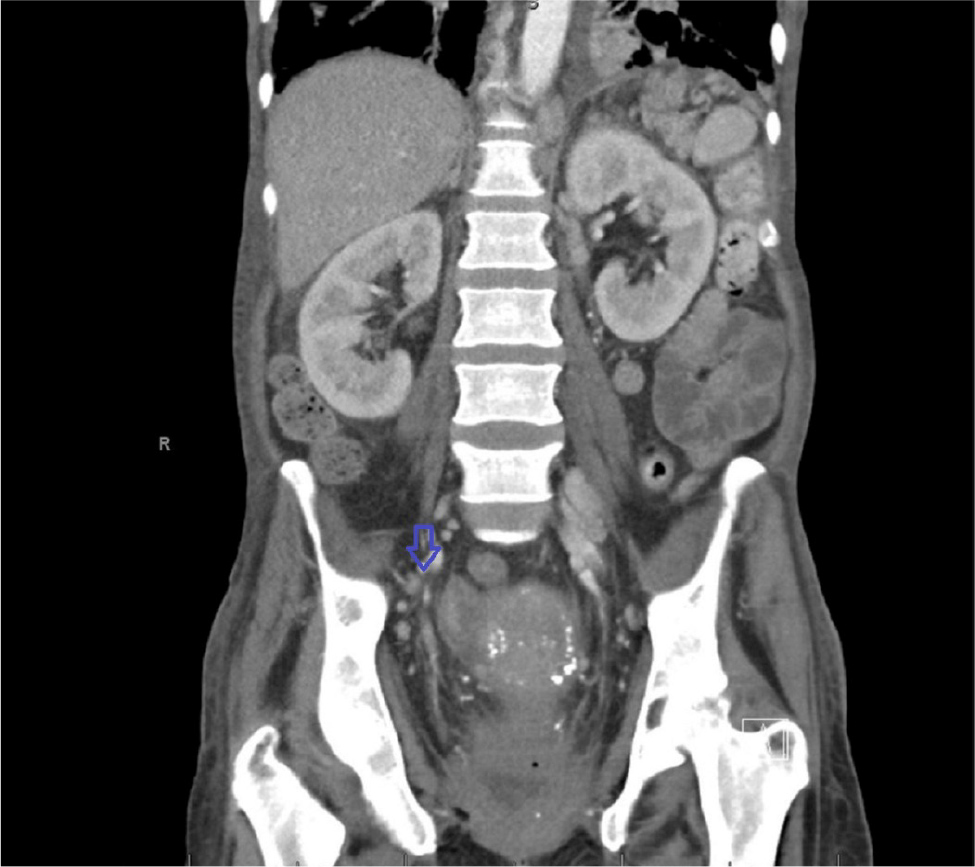
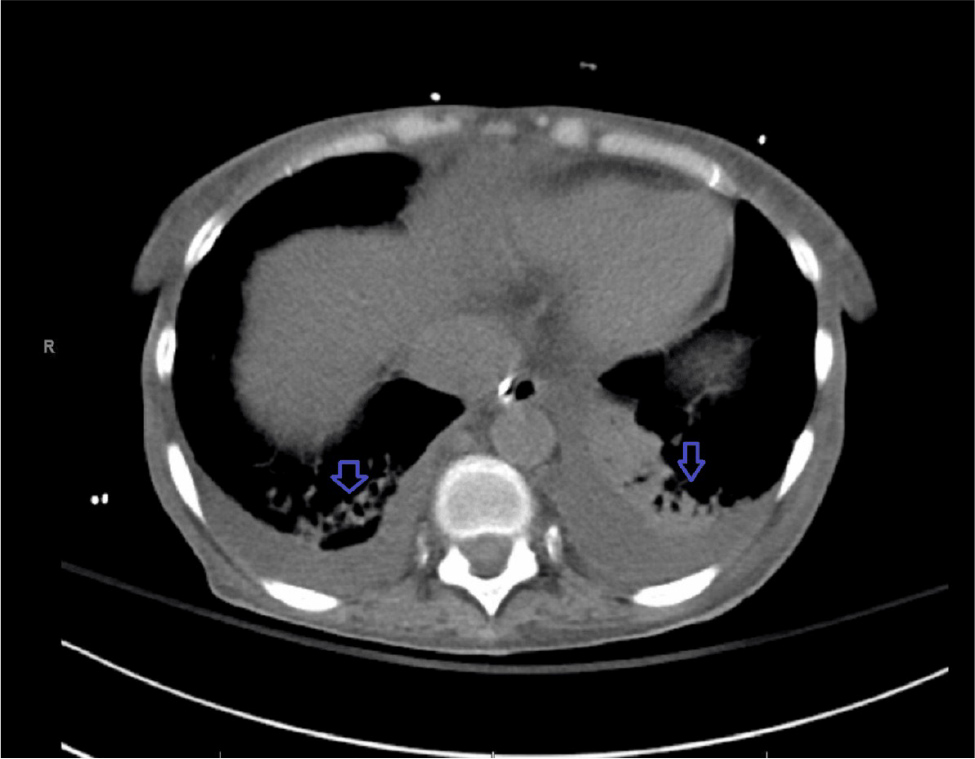
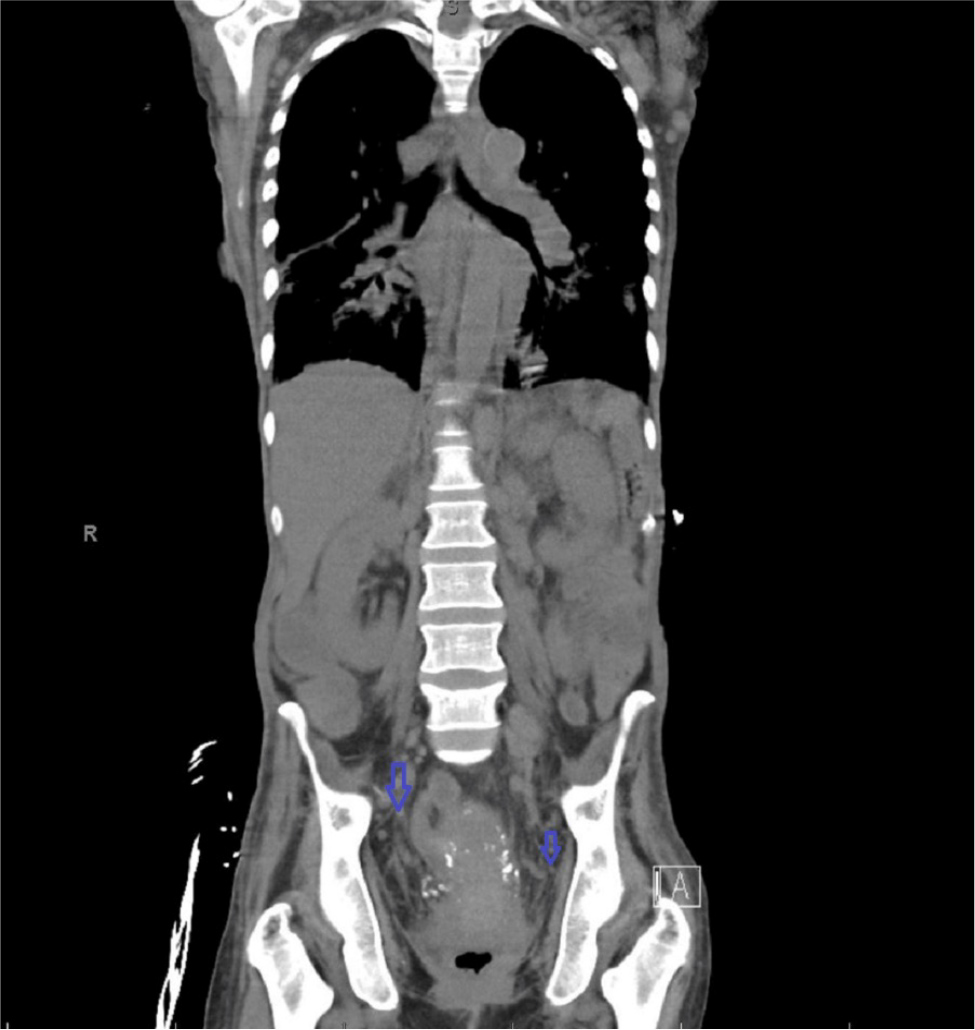
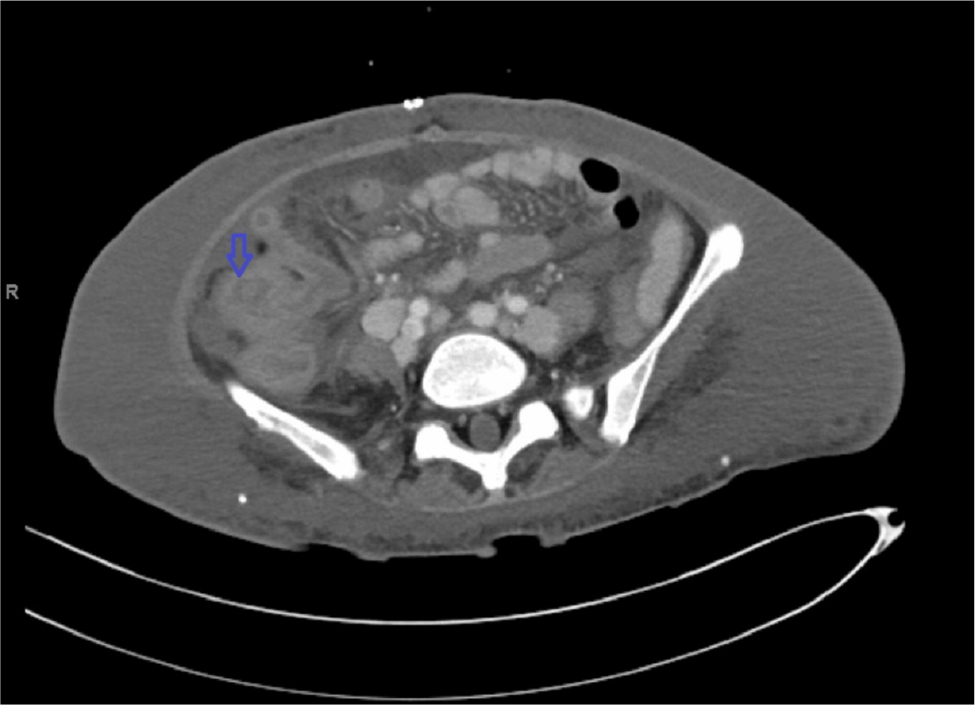
The patient received packed red blood cell and fresh frozen plasma, transfusions as well as intravenous fluid in the ED. She was started on meropenem and vancomycin and was admitted to the general medical floor. Given her severe acute kidney injury and metabolic acidosis, she was started on continuous renal replacement therapy (CRRT). Computed tomography (CT) scan of the abdomen and pelvis showed retroperitoneal, bilateral iliac chain and bilateral inguinal lymphadenopathy (Fig. 1). Nodules were also noted at the lung bases raising concern for malignancy. An axillary lymph node biopsy was done which showed an atypical lymphoid infiltrate. The patient continued to deteriorate and required intubation, initiation of vasopressors and admission to the intensive care unit (ICU). Repeat CT scan of the chest, abdomen and pelvis showed basilar fibrotic lung disease (Fig. 2), multiple pulmonary nodules, severe mediastinal, axillary, retroperitoneal and inguinal lymphadenopathy worse than in the previous scan (Fig. 3), gallbladder distension and thickening of the rectal wall and sigmoid colon (Fig. 4). A cholecystostomy tube was placed. The procedure was complicated by hemoperitoneum which required an exploratory laparotomy to control liver bleeding. Open cholecystectomy was also performed. Omental lymphadenopathy was noted intraoperatively and a biopsy was sent which showed B-cell lymphoma. The patient continued to have lactic acidosis and was switched from CRRT to hemodialysis. Given her continued encephalopathy she could not be weaned off mechanical ventilatory support. Despite multiple calcium replacement while in the ICU, the patient’s ionized calcium level continued to decline. On day 17 of her hospital stay the patient developed torsade de point and cardiac arrest. At the family’s request her code status was changed to comfort measures only and she expired.
Figure 2. CT scan of the chest showing bibasilar fibrotic lung disease (blue arrows).
Figure 3. Computed tomography scan coronal view showing inguinal lymphadenopathy (blue arrows) worse comparing to Figure 1.
DISCUSSION
Hypocalcemia, defined as a low level of calcium in the blood, has substantial implications for the cardiovascular system, potentially precipitating arrhythmias, and cardiac arrest[1]. The clinical manifestations of hypocalcemia vary, correlating with the level of calcium and acuity of the condition. Symptoms include neuromuscular excitation (presenting as anxiety, delirium, depression, and coma) as well as cardiovascular effects including hypotension, cardiomyopathy, and arrhythmias[4,5].
The cardiovascular side effects are of particular concern, as they may lead to refractory hypotension and impaired myocardial contractility, with resistance to vasopressor therapy. Additionally, hypocalcemia can induce ST segment prolongation on an ECG, manifesting as QT prolongation with a normal-sized T-wave. This ECG alteration is associated with an elevated risk of torsades de pointes and subsequent cardiac death[3-5]. Instances of cardiac arrest attributable to hypocalcemia-mediated arrhythmias are well-documented in the literature[6]. Heart block and bradycardia are among the potential arrhythmic manifestations. Severe hypocalcemia can further trigger the release of substantial amounts of phosphate from lysed muscle cells which, if patients survive, may increase the eventual risk of death from cardiovascular disease related to vascular calcification[7-10]. In conclusion, hypocalcemia poses a significant threat to the cardiovascular system, culminating in cardiac arrest and arrhythmias. Vigilant monitoring of calcium levels is imperative in patients with suspected hypocalcemia, particularly those undergoing massive transfusion, experiencing known arrhythmia or seizures, or exhibiting general electrolytic disarray. Early identification and appropriate management of hypocalcemia hold the potential to avert these severe cardiovascular complications.