ABSTRACT
Introduction: Renal squamous cell carcinoma (SCC) is a neoplasm with an extremely rare occurrence compared to other renal malignancies. The classic presentation includes a palpable mass and flank pain; however, the presentation is seldom non-specific. Our study describes the significance of programmed death ligand-1 (PD-L1) expression in renal cancer and its association with clinical outcomes, alongside available treatment options.
Case description: An 80-year-old female with a history of hypertension and cerebral aneurysm presented with right flank pain and blood in urine and was diagnosed with pyelonephritis and left renal mass/phlegmon. A biopsy revealed SCC of the kidney with metastasis to the lung and aortocaval lymph node. Positron emission tomography (PET) scan confirmed malignancy in the kidney and lung. Treatment with pemrolizumab and carboplatin plus paclitaxel was initiated but poorly tolerated as the haemoglobin dropped rapidly.
Conclusion: SCC poses a diagnostic challenge due to its rarity and non-specific symptoms, often leading to advanced stage diagnosis. PD-L1 expression is pivotal in assessing tumour aggressiveness and prognosis. PD-L1 inhibitors offer promise, but their efficacy in renal SCC warrants further investigation. Radical nephrectomy and systemic chemotherapy show potential in advanced cases, necessitating vigilant management of treatment-related side effects. This case emphasises the need for ongoing research to refine therapeutic approaches and enhance outcomes in renal SCC patients.
KEYWORDS
Squamous cell carcinoma, renal cancer, PD-L1, pembrolizumab, neoplasm
LEARNING POINTS
- PD-L1 expression is pivotal in assessing tumour aggressiveness and prognosis of renal cell carcinoma.
- PD-L1 inhibitors hold promise as a therapeutic intervention in renal squamous cancer.
- Radical nephrectomy and systemic chemotherapy show potential in managing advanced renal cancer.
INTRODUCTION
Renal squamous cell carcinoma (SCC) is a neoplasm with an extremely rare occurrence compared to other renal malignancies[1]. It typically presents in older adults (over 50 years). It occurs as a result of squamous metaplasia of the urothelium lining the renal collecting system, usually stemming from chronic irritation and infection, a common sequela of nephrolithiasis[2]. Due to the covert symptoms and radiographic findings, renal SCC mostly remains hidden until the later stages, when symptoms become apparent. While some studies hint at a female predilection, conflicting reports cloud the precise gender distribution[3]. The classic presentation includes haematuria, flank pain and a palpable mass; however, literature reports a wide spectrum of manifestations including hydronephrosis, pyelonephritis and hypercalcaemia secondary to paraneoplastic syndromes[4,5].
The rare nature of the neoplasm presents several diagnostic challenges due to the unpredictable presentations and non-specific radiological findings, which can delay diagnosis until a histopathological examination. The carcinoma is also characterised by early metastasis, accounting for its poor prognosis[6]. We present the case of an 80-year-old female with renal SCC and a medical history including hypertension, cerebral aneurysm and endarterectomy. Furthermore, this study explores the significance of programmed death ligand-1 (PD-L1) expression in renal cancer and its association with clinical outcomes, alongside available treatment options. We further delve into the potential of PD-L1 inhibitors in managing renal SCC, as well as other treatments available.
CASE DESCRIPTION
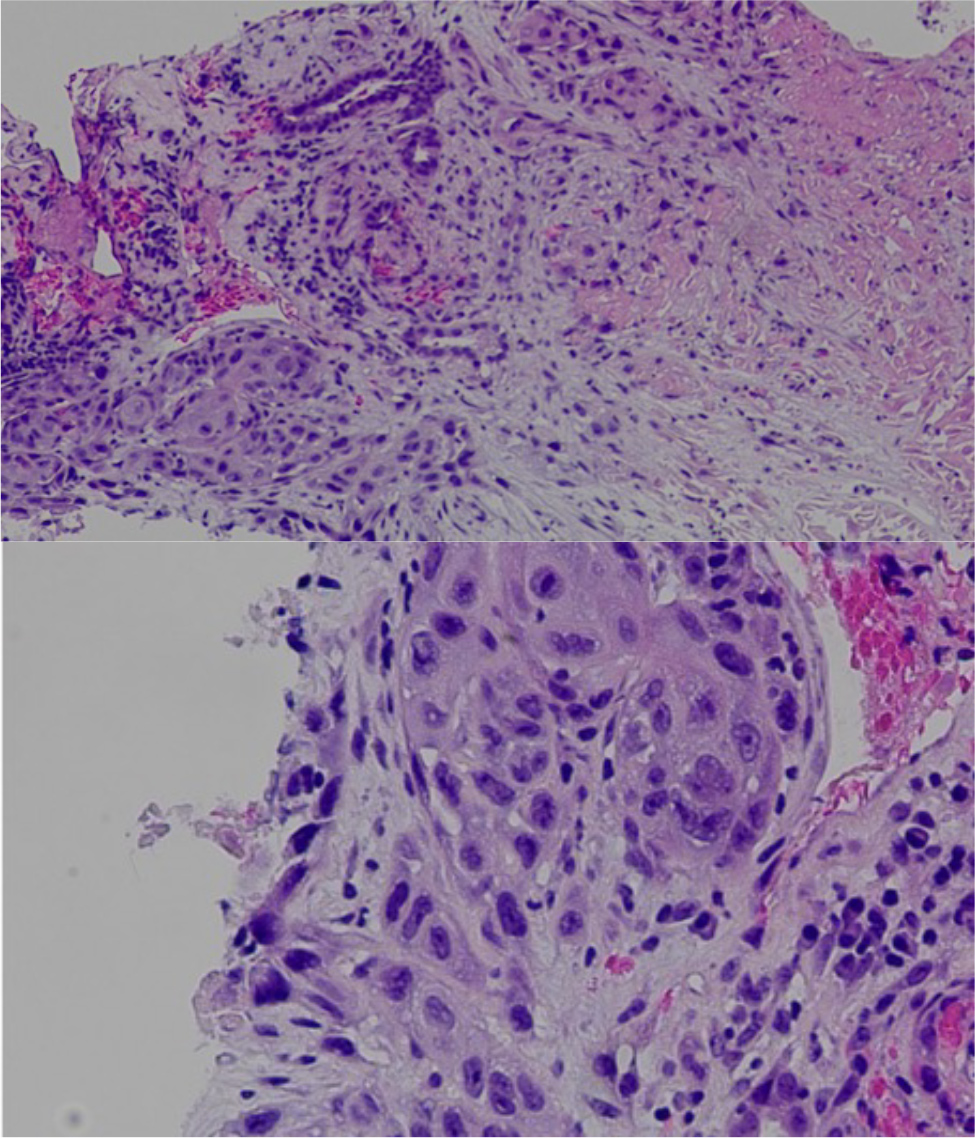
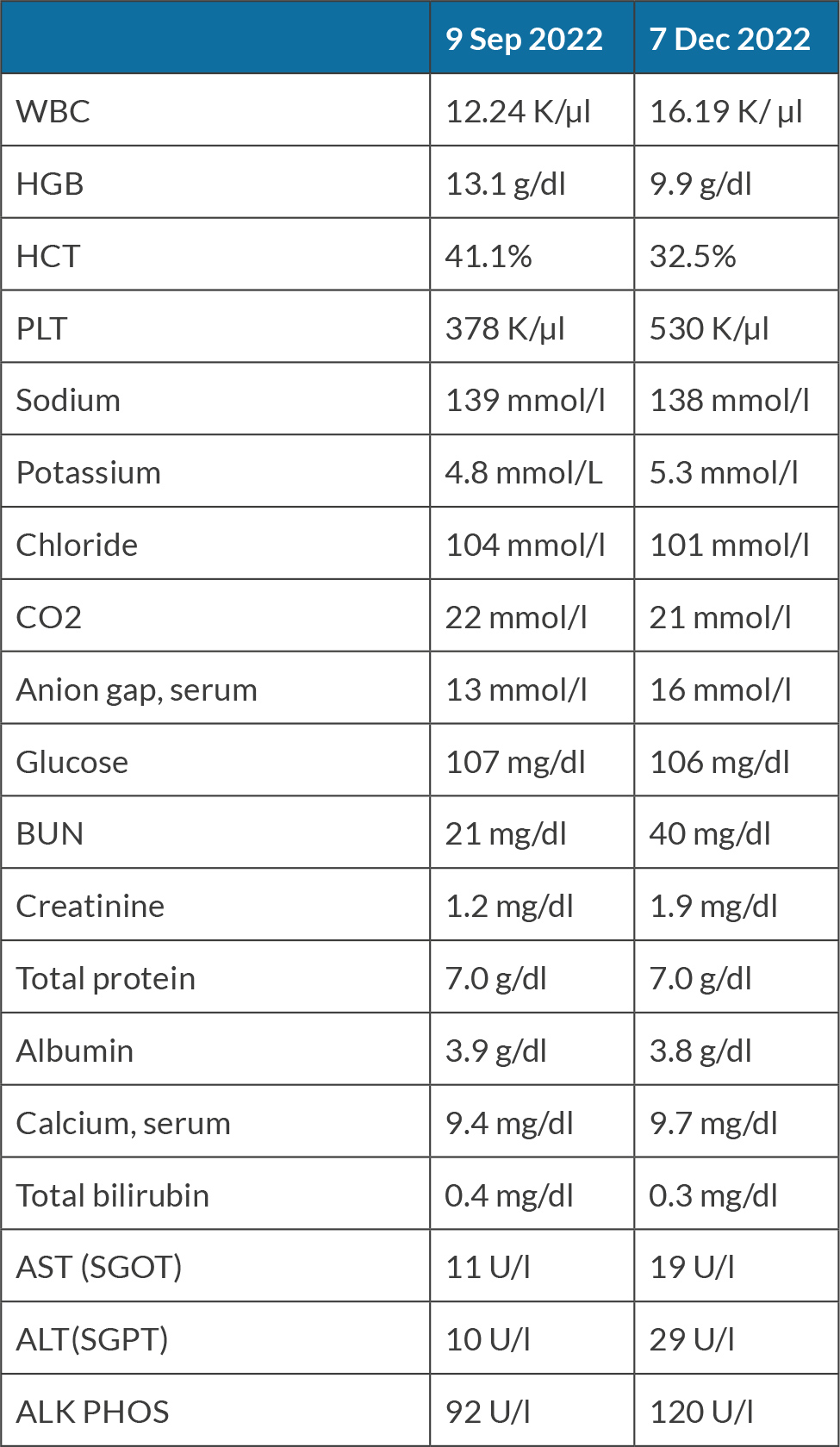
An 80-year-old female with past medical history of hypertension, cerebral aneurysm clipping, and bilateral endarterectomy presented to the clinic for consultation of right flank pain. The patient mentioned that about 8 weeks previously, she woke up during the night with sudden onset of right flank pain and noticed blood in the urine, which prompted her to go to the emergency room (ER). In the ER she had a computerized tomography (CT) scan of the abdomen that showed findings consistent with pyelonephritis and left renal mass phlegmon. During the hospitalisation she was treated with intravenous antibiotics for pyelonephritis and was discharged home with antibiotics. She was advised to follow up with Haematology and Oncology, with planned follow-up CT scans and biopsy of the mass. The pathology report showed that the mass was consistent with SCC of the kidney. The biopsy showed renal parenchyma infiltrated by keratinising SCC associated with extensive necrosis. No definitive urothelial component was present, although this does not completely rule out urothelial carcinoma with squamous differentiation (Fig. 1). Later, the patient also underwent a positron emission tomography (PET) scan which showed a hypermetabolic right kidney mass proving the biopsy malignancy. 18-Fluoro-deoxyglucose positron emission tomography (FGD-PET) also showed right lung nodules concerning for metastasis. On the PET scan there was hypermetabolic activity in the aortocaval lymph node in the retroperitoneum, consistent with nodal metastasis. The biopsy of the lung pulmonary nodule failed to prove malignancy. The magnetic resonance imaging (MRI) head was negative for any intracranial metastasis. The patient was informed regarding the diagnosis, prognosis and options for management including but not limited to nephrectomy with or without urethrectomy. The patient was started on pemrolizumab every three weeks along with carboplatin plus paclitaxel combination, but this was tolerated very poorly as haemoglobin dropped from 9.7 to 7.5 in one week. The patient’s laboratory investigations over time are summarized in Table 1, showing significant changes in haematological parameters and kidney function.
Figure 1. Keratinising squamous cells with extensive necrosis; findings consistent with renal SCC.
DISCUSSION
Primary renal SCC is a rare entity, accounting for around 0.6% of all renal malignancy[1]. For renal SCC, following nephrectomy, patients achieving disease-free status face the highest risk of recurrence within the initial 5 years of being disease-free. The recurrence often presents with distal metastasis, which necessitates systemic therapy. The role of programmed death 1 (PD-L1) in renal SCC and its treatment through programmed death ligand 1 (PD-L1) inhibitors have garnered significant attention in its efficacy against different malignancies, specifically cancers in advanced stages. Its expression has been reported in a subset of renal SCC samples and is associated with a more aggressive tumour behaviour and poor prognosis. PD-L1, also known as B7-H1 or CD274, is markedly expressed in various tumour types and serves as a robust prognostic indicator for affected individuals. PD-L1 binding to its receptor PD-1 on activated T lymphocytes and other immune cells, exerts a negative regulation on T-cell proliferation and activity. This interaction enables tumour cells to evade immune surveillance and clearance. Studies have revealed that positive PD-L1 expression is associated with an advanced tumour stage, regional lymph node metastasis and distant metastasis, highlighting its potential as an adverse prognostic factor for both progression-free survival and overall survival[2]. Positive PD-L1 expression has been associated with poorer prognosis in multiple malignancies, particularly those arising from epithelial tissues such as oesophageal cancer, gastric cancer and oropharyngeal SCC. Thompson et al.[7] were among the pioneers in elucidating the clinical relevance of PD-L1 expression in renal cell carcinoma (RCC) patients through immunohistochemical techniques.
Pembrolizumab, an immune checkpoint inhibitor, is a PD-1 blocking agent[3]. It is an IgG4 kappa isotope anti-PD1 monoclonal antibody that helps revive the immune response against cancer cells. Immune checkpoints are cellular proteins, which function as brakes on the immune system. The rationale behind the empiric treatment with pembrolizumab in renal SCC stems from the high expression of PD-1 in this cancer type. The drug binds to and blocks PD-1 on the surface of T cells, thereby preventing PD-L1 from binding to its receptor, PD-1. This helps restore the immune surveillance and eventually combats tumour progression. Notably, the KEYNOTE-564 study, a landmark randomised phase 3 trial, marks a significant milestone in adjuvant immunotherapy for RCC patients[3]. This trial compared adjuvant pembrolizumab monotherapy with a placebo in participants with localised and metastasised renal cancer[3]. The study regimen involved administering pembrolizumab 200 mg intravenously every 3 weeks, for up to 17 cycles or until progression or recurrence. Results from the trial revealed a noteworthy improvement in disease-free survival with pembrolizumab compared to the placebo at the 24-month follow-up, which was clinically meaningful and statistically significant[3]. Furthermore, an extended 6-month follow-up analysis again demonstrated improved overall survival with pembrolizumab, reinforcing the previous findings[3]. Additionally, pembrolizumab exhibited enhanced distant metastasis-free survival compared to the placebo[3]. Concerning adverse effects, pembrolizumab was associated with a higher incidence of serious side effects (20.7 vs. 11.5% with a placebo), although no fatalities were attributed to the therapy[3]. Fasano et al.[4] documented a case illustrating various toxicities associated with pembrolizumab, encompassing neurological, rheumatological, renal and endocrine manifestations. Renal toxicity primarily stems from drug-induced interstitial nephritis, potentially leading to acute kidney injury[5]. These adverse effects are compounded by the necessity for aggressive management modalities such as high-dose steroid administration, plasmapheresis and – in severe cases – Intensive Care Unit admission, further emphasising the complexities associated with the use of these agents.
However, given that the KEYNOTE trial had not accrued the requisite number of events for final analysis by the data cut-off date, further follow-up analysis is essential to supplement the lack of mature overall survival data. The lack of therapeutic benefit observed with pembrolizumab in our patient demonstrates the necessity for a more nuanced understanding of its effectiveness, particularly in the context of renal SCC. Notably, while pembrolizumab was evaluated in the KEYNOTE-564 trial for the clear cell subtype of RCC, the underlying mechanism of action remains consistent across both subtypes. Despite this, the trial’s outcomes suggest promising results, positioning pembrolizumab as a potential new standard of care for renal cancer patients, especially those at heightened risk of recurrence post-surgery. Our case serves as a poignant reminder of the complexities in achieving therapeutic success. Previously, vascular endothelial growth factor (VEGF) tyrosine-kinase inhibitors have shown potential as adjuvant agents in advanced RCC[4] and its use has been extensively investigated[5]. However, studies have consistently indicated that these agents, including pazopanib, sorafenib and axitinib, did not substantially enhance the efficacy of adjuvant therapy in advanced disease[5]. Despite sunitinib’s approval for adjuvant use in RCC, compelling evidence supporting its efficacy in this context remains sparse. Overall, these findings support the lack of significant survival benefit associated with VEGF tyrosine-kinase inhibitors when employed as adjuvant therapy in advanced RCC. Concerning PD-1 blocking agents, the existing data is insufficient to definitively establish their efficacy, particularly in advanced renal SCC stages. Concurrently, keeping in view the current immune escape mechanisms used by renal SCC cells in the developing tumour neoantigens and evading treatment, the need for more advanced therapies becomes evident, especially in tumours with unique receptor expression. For instance, the clonal proliferation of tumour cells, showcasing specific human leukocyte antigen subtypes, impedes antigen expression, thereby evading T-cell recognition and apoptosis[2].
Radical nephrectomy with lymph node resection can offer curative potential in the early stages of renal SCC, particularly when the tumour has not invaded extensively, and chemotherapy has not been extensively documented. Notably, Chang et al.[2] reported favourable outcomes from systemic chemotherapy in an individual with advanced RCC, for whom surgery was contraindicated. Subsequent imaging post-chemotherapy revealed significant tumour remission, culminating in successful surgery to completely resect the mass. This shows the potential efficacy of chemotherapy as a prelude to surgery. However, while radical nephrectomy and systemic chemotherapy exhibit promise in managing advanced disease, vigilant monitoring and management of treatment-related toxicities are imperative. Nonetheless, the observed inefficacy of pembrolizumab in our case highlights the intricacies associated with treating renal SCC. The failure of pembrolizumab prompts speculation regarding various factors potentially influencing treatment outcomes. It is plausible that the concurrent administration of other medications, such as carboplatin and paclitaxel, alongside the patient’s age (80) and the advanced cancer stage (notably with lung metastasis) could have contributed to the ineffectiveness of pembrolizumab. Importantly, the clinical trial evaluating pembrolizumab[3] primarily focused on tumours in their initial stages with no metastasis, rather than more advanced stages akin to our patient’s condition. This disparity in disease stage may have influenced treatment response. Therefore, careful consideration of treatment strategies and ongoing research efforts are essential for advancing therapeutic options and enhancing patient care in this challenging clinical scenario. Understanding the significance of PD-L1 expression in renal SCC is pivotal, given its association with aggressive tumour behaviour and inferior prognosis. While PD-L1 inhibitors hold promise as a therapeutic avenue, further research is indispensable to delineate their efficacy in treating renal SCC comprehensively.
CONCLUSION
This case report demonstrates the imperative of ongoing research and development of innovative therapeutic strategies to improve outcomes for patients with renal cancer. Further investigation into the role of immune checkpoint inhibitors – especially PD-L1 inhibitors – and alternative therapeutic modalities is warranted to augment these findings and furnish robust clinical evidence.