ABSTRACT
Pneumocystis jirovecii is an opportunistic fungus that infects the lungs but can involve other organs, including the skin and lymph nodes. Risk factors include human immunodeficiency virus (HIV), solid organ/haematological malignancies and a CD4 cell count of fewer than 200 cells/µl. Pneumocystis jirovecii pneumonia (PJP) infection is reported less frequently these days with the advent of prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMX).
We report a case of extrapulmonary PJP infection in a patient while receiving pentamidine prophylaxis in a T-cell prolymphocytic leukaemia, who underwent an allogeneic stem cell transplant. There are plenty of reported cases of PJP on pentamidine prophylaxis; however, none had cutaneous PJP infection. Cutaneous P. jirovecii infection (CPJ) is an extrapulmonary infection that is rarely reported. Our patient’s skin biopsy was inconclusive, but the skin nodules improved once he was initiated on TMP-SMX. Many transplant patients cannot tolerate TMP-SMX for various reasons and are placed on second-line prophylaxis for PJP, which does not prevent extrapulmonary PJP infections. Our case highlights the challenges of diagnosing such a rare infection in immunocompromised patients. Extrapulmonary PJP should be suspected in patients with a history of pulmonary PJP and persistent elevated Fungitell® levels in low CD4 counts.
KEYWORDS
Cutaneous pneumocystis, stem cell transplant
LEARNING POINTS
- Extrapulmonary Pneumocystis jirovecii pneumonia (PJP) infection can happen while receiving pentamidine prophylaxis.
- It is extremely rare to see a cutaneous infection, and no case has been reported in the last two decades.
- Trimethoprim-sulfamethoxazole (TMP-SMX) remains the first-line treatment for pulmonary and extrapulmonary PJP.
INTRODUCTION
Pneumocystis jirovecii is an opportunistic fungal pathogen that mostly causes severe pneumonia and, rarely, involves other organs in immunocompromised hosts. Risk factors include leukaemia, HIV, organ transplant, malignancy, certain inflammatory or rheumatologic conditions, and associated therapies and conditions that result in cell-mediated immune deficiency[1]. Limited cases of cutaneous Pneumocystis jirovecii pneumonia (PJP) are reported in the literature, mostly from the HIV era. We present a disseminated (respiratory and cutaneous) PJP case in a leukaemia post-allogenic stem cell transplant patient that improved with TMP-SMX treatment.
CASE DESCRIPTION
A 67-year-old male patient with T-cell prolymphocytic leukaemia who underwent an allogeneic stem cell transplant (SCT) had multiple issues post-transplant. These included prolonged leukopenia, a CD4 count of less than 200 cells/µl, graft versus host disease (GVHD), and other opportunistic infections such as cytomegalovirus (CMV), John Cunningham, and SARS-CoV-2. The patient was initially placed on TMP-SMX prophylaxis post-transplant, but this was held due to kidney stones, renal failure and low counts. He was kept on inhaled pentamidine. According to institutional protocols, the patient was initially placed on immunoprophylaxis with posaconazole and valacyclovir. For GVHD prophylaxis, he was initiated on cyclophosphamide on days 3 and 4, and rabbit ATG on day 5. He developed grade II stage I GI GVHD, which was treated with beclomethasone and budesonide. He was kept on sirolimus with tacrolimus post-transplant and continued for six months until he was deemed low risk for GVHD.
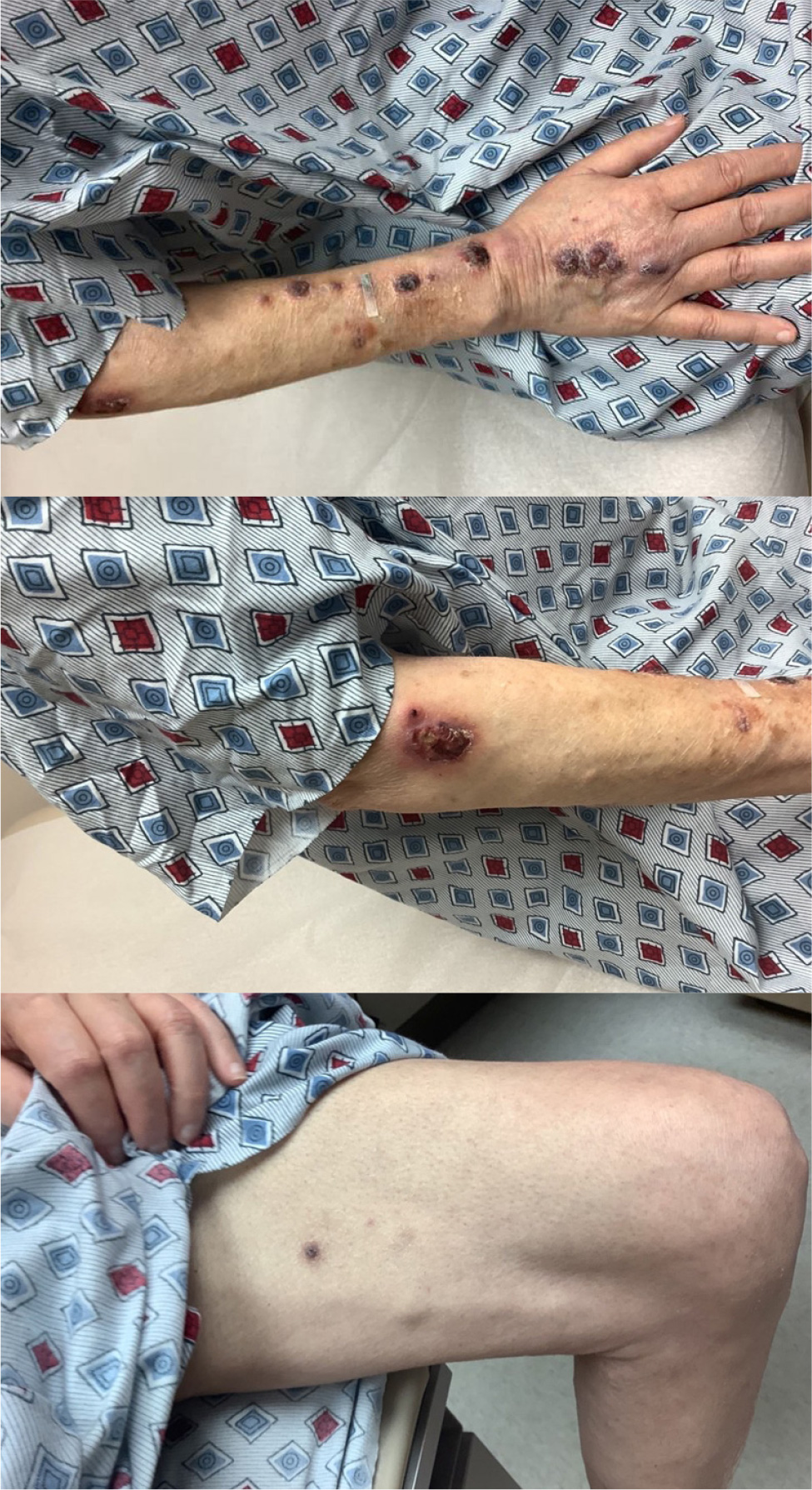
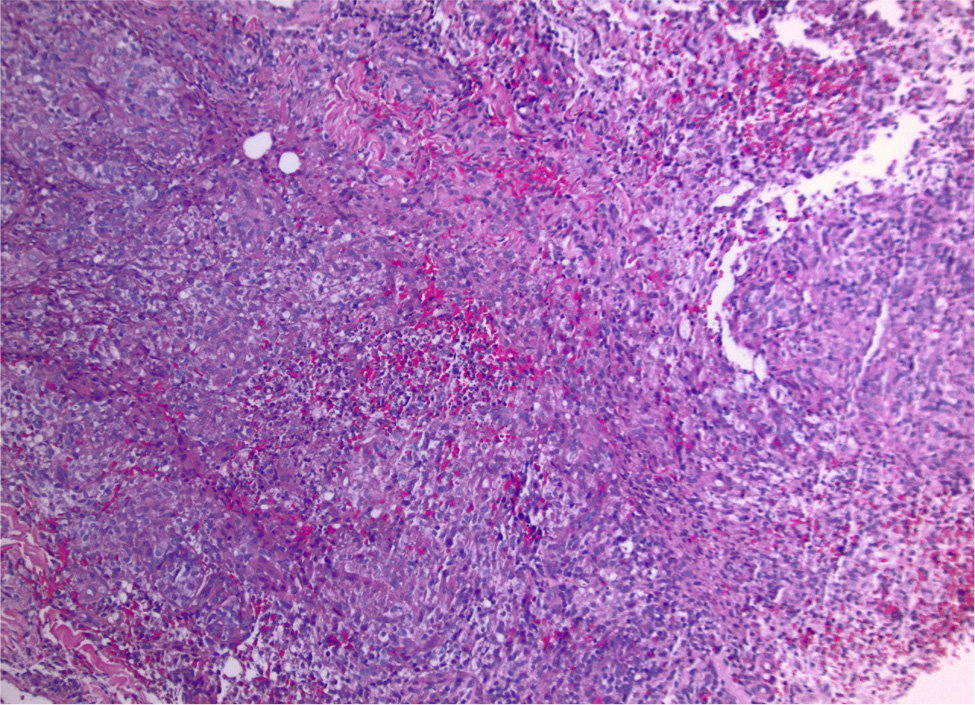
Six months post-transplant, the patient presented to the emergency room with fever, dyspnoea, and cough. The initial work-up included sputum cultures, a respiratory viral panel and a SARS-CoV-2 swab, which returned negative results. A month prior, he was initiated on valganciclovir for a CMV infection. A bronchoscopy with biopsy returned positive for PJP and CMV. He was initiated on TMP/SMX with steroids. He required up to 3 litres of oxygen initially but improved clinically and was discharged home on TMP-SMX for three weeks. Prophylactic TMP-SMX was continued after that. Six weeks later, the patient presented with multiple nodular lesions on the forearms, ankles and legs (Fig. 1). They were 1–2 cm painless, non-itchy, reddish nodules with elevated edges and focal ulceration. A punch and deeper skin biopsy showed suppurative granulomatous inflammation, which was suspected to be infectious. Special stains, including mycobacterial and fungal stains, were negative. (Fig. 2). There was no evidence of GVHD or leukaemia. The Fungitell® level rose above 500 pg/ml from 110 pg/ml a month previously; he had no respiratory symptoms at this time. Due to persistently elevated Fungitell® levels and a CD4 count of less than 50, it was concluded that the skin lesions were consistent with CPJ after a discussion between dermatopathology, haematology and infectious disease. The patient was initiated on therapeutic doses of TMP-SMX. Within a few days, Fungitell® levels became undetectable, further supporting the diagnosis of CPJ. The patient was treated with therapeutic doses of TMP-SMX for six weeks, with close follow-ups. Over the next few weeks, the patient showed steady improvement with near resolution of his skin lesions.
Figure 1. Arm and leg nodules showing various stages of 1–2 cm painless, non-itchy, reddish nodules with elevated edges and focal ulceration.
Figure 2. A skin punch biopsy showed a dense dermal suppurative granulomatous infiltrate. Special stains (AFB, Giemsa, PAS, GMS, Fite) were negative for organisms, but the findings were most compatible with an infectious process. There was no evidence of GVHD or a leukaemic process.
DISCUSSION
CPJ is an extrapulmonary manifestation of disseminated pneumocystis infection that has become rare due to the widespread use of TMP/SMX for PJP prophylaxis in immunocompromised hosts. Although more than 100 cases of extrapulmonary infections were reported before 1997, only five cases have been reported over the last two decades, and all five of those patients were HIV positive[2]. The presentation of CPJ can be multiple purple papules and nodules that can be scattered on the torso, arms, legs and orbital rim[3]. They can mimic Kaposi sarcoma, which is seen in HIV patients[4]. A skin biopsy may confirm the diagnosis, but it could be inconclusive, as in our case. Our literature review did not reveal reported cases of CPJ in SCT patients. In our case, the supportive findings of low CD4 count, persistently elevated Fungitell® levels in the absence of respiratory symptoms, the absence of GVHD and leukaemia cells on skin biopsy, similar pathologic findings in skin biopsy with bronchoalveolar lavage, lack of an alternate diagnosis and improvement with therapeutic doses of TMP-SMX indicated possible CPJ. Although Pneumocystis-mediated infection rarely causes extrapulmonary manifestations, such findings may be present in patients receiving aerosolised pentamidine for prophylaxis, or in patients with advanced HIV infection who are not taking any prophylaxis[5,6]. One interesting feature of PJP is that in non-HIV patients PJP presents more acutely, causing severe respiratory illness with rapid deterioration, and it carries higher mortality with poor prognosis compared to HIV patients where the illness has a slower course with subacute presentation and better prognosis. The impact of PJP on morbidity and mortality in immunocompromised patients is substantial. Up to 40% of patients with acute lymphoblastic leukaemia or lymphoproliferative diseases become infected with PJP unless systemic prophylaxis is given[7]. In SCT recipients, PJP is associated with CMV pneumonia in around 50% of cases[7,8]. Case reports indicate that PJP can recur in patients who receive inhaled pentamidine and that clinical features may be atypical[9]. Physical examination is usually non-specific, even with significant disease and hypoxaemia. Chest imaging findings in patients receiving inhaled pentamidine include cavities, pneumothoraces, bilateral and upper lobe interstitial infiltrates, and pleural effusion. A diagnosis can be established by demonstrating eosinophilic foamy exudates with P. jirovecii cystic form (ascus) or trophic form in affected tissue by Grocott-Gomori methenamine silver (GMS) staining or periodic acid-Schiff (PAS) staining[3]. Testing for blood 1-3-β-D-glucan levels can also help diagnose PJP. Serum Fungitell® has a sensitivity of 80% and specificity of 63%, making it a decent initial test to direct further testing. When there is adequate suspicion for PJP, the detection of serum 1-3-β-D-glucan may be helpful for assurance in starting antimicrobial therapy empirically[9]. Immunocompromised individuals without HIV may have elevated serum lactate dehydrogenase; therefore, this is not a highly valuable diagnostic tool in this patient population[9]. Detecting PJP by polymerase chain reaction assay, which is more sensitive than GMS staining, may help confirm the diagnosis, especially in patients with uncommon manifestations[3,10]. TMP/SMX remains the first-line treatment for both pulmonary and extrapulmonary PJP. TMP/SMX is not well tolerated in haematopoietic stem cell recipients, with reported intolerance as high as 55% requiring discontinuation of the drug due to rash, marrow suppression, renal failure, and allergy[7,8]. There is no consensus regarding the efficacy of second-line agents in SCT patients nor an appropriate algorithm for choosing among second-line agents[11]. A recent meta-analysis concluded monthly IV pentamidine to be a good second-line agent[12]. PJP breakthrough rates can be up to 9% for aerosolised pentamidine, ~7% for dapsone with several reported failures with these agents, although these account for less than 300 total reported patients[8]. Duration of treatment is typically three weeks, and secondary PJP prophylaxis is indicated in all patients after that for up to 6 months or till the CD4+ lymphocyte count improves above 200 cells/µl[13]. Immunoprophylaxis with inhaled pentamidine has been suggested as a risk factor for extrapulmonary PJP due to inadequate concentration of aerosolised pentamidine to suppress systemic infection[8].
CONCLUSION
In summary, it is challenging to manage SCT patients because of concurrent viral and fungal co-infections. A lot of transplant patients cannot tolerate TMP-SMX for various reasons and are placed on second-line prophylaxis such as pentamidine for PJP. This does not prevent extrapulmonary PJP infections. Extrapulmonary pneumocystis in a cutaneous form is very rare. Our case highlights the importance of staying vigilant for extrapulmonary manifestations of PJP in patients with a history of pulmonary PJP and persistent elevated Fungitell® levels in low CD4 counts.