ABSTRACT
Bronchial artery embolization (BAE) is a procedure that aims to control bleeding from bronchial arteries in massive and chronic haemoptysis. It is considered to be a life-saving measure in severe life-threatening haemoptysis. Although it is minimally invasive and has a high success rate, it still carries a list of complications. These include post-embolisation syndrome, chest pain, back pain, dysphagia, vascular injury at the site of the embolisation leading to dissection, perforation, pseudoaneurysm and, very rarely, embolic infarction to non-target vessels.
Stroke is one of the rare complications post BAE, and it can be severe and fatal. Few cases of stroke post BAE have been reported in the literature, and they were mainly due to posterior cerebral circulation infarction. Here, we report a case of a stroke post BAE due to massive middle cerebral artery (MCA) infarction and to our knowledge it seems to be the first reported case of MCA infarction post BAE.
The discussion will cover the possible mechanisms of embolic passage, the outcome of the case including rehabilitation perspectives and the learning points. These will also highlight the importance of early recognition, which can save patients from a disabling stroke in the future.
KEYWORDS
Pulmonology, neurology, rehabilitation, bronchial artery embolisation, stroke
LEARNING POINTS
- Bronchial artery embolisation (BAE) carries a high risk of significant complications such as transverse myelitis, bronchial infraction, ischaemic colitis and stroke. While stroke remains one of the rarest complication post BAE, it may be under-reported or unrecognised.
- Close monitoring in post-BAE patients for any abnormal neurological signs that warrant urgent brain imaging, and early recognition can save patients from a disabling stroke by having the appropriate hyperactive stroke management plan including mechanical thrombectomy.
INTRODUCTION
Massive haemoptysis is a rare, life-threating emergency with a high morbidity and mortality. It is defined as blood loss > 500 ml in 24 hours. Life-threatening events include significant airway obstruction, asphyxia, abnormal gas exchange and haemodynamic instability. Most of the life-threatening haemoptysis arises from the high-pressure bronchial artery circulation. Common aetiology includes bronchiectasis, pulmonary tuberculosis, bronchogenic carcinoma and fungal infection.
Bronchial artery embolisation (BAE) is the gold-standard treatment. After initial stabilisation and bronchoscopy, the patient is transferred to the radiological suite. During arteriography, a contrast is injected into the bronchial circulation, and a suspicious area is identified. Contrast extravasation into a specific area is very rare, therefore sound clinical judgement is required during arteriography to identify abnormal vascular structure, torturous vessels or a focal hyper-vascularised area. Occlusive material (such as coils, vascular plugs) is then injected into the suspected area, or proximal to the supplying vasculature to ensure homeostasis.
BAE has a high success rate of 85–100%[1] and has almost replaced surgical treatment, which carries a high risk of complications. It is minimally invasive, with fewer complications. Adverse effects include chest pain (most common), dysphagia, bronchial or aortic dissection[2], post-embolisation syndrome (fever with high white blood cell count)[3] and non-target embolisation (rare but can be fatal)[3]. Failure or delay in early assessment can further result in increased intracranial pressure, brain stem herniation and even death.
By reviewing PubMed literature from 2005 to 2023 using the keywords: ‘Bronchial artery embolization’, ‘complications’, ‘cerebral infarction’, ‘stroke’, ‘non-target embolization’, ‘pathology’, ‘middle cerebral artery infarction’ and ‘preventive measures’, we found complications of non-target embolisation including myocardial infarction[4], ischaemic colitis[3], anterior spinal artery infarction[5] and posterior cerebral artery infarction[6-10]. The search included all papers and studies that discussed the possible complications of BAE, specifically non-target embolisation to the cerebral circulation, and excluded any other papers that discussed other complications not related to our aims of discussion.
Yu et al.[10] have reported 14 cases with cerebral infarction as a complication of BAE, where the posterior circulation was mostly affected. Here, we report a case of massive MCA infarction after BAE, and to our knowledge this is the first case to be reported.
CASE DESCRIPTION
A 38-year-old native Syrian male, with a background of bronchiectasis presented to our emergency room with two episodes of massive haemoptysis and had coughed up more than 500 ml of fresh blood in total.
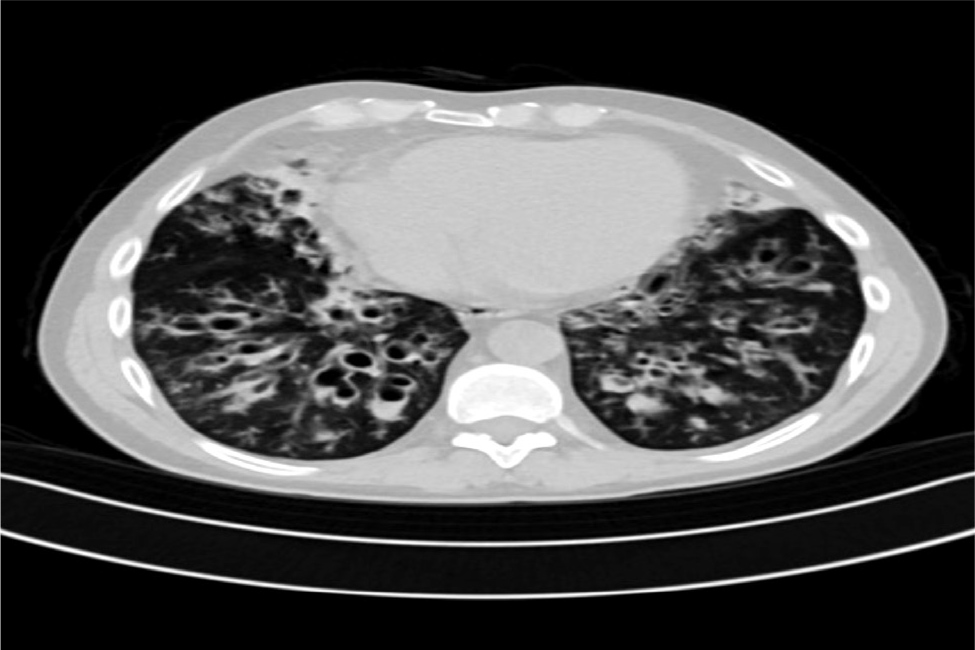
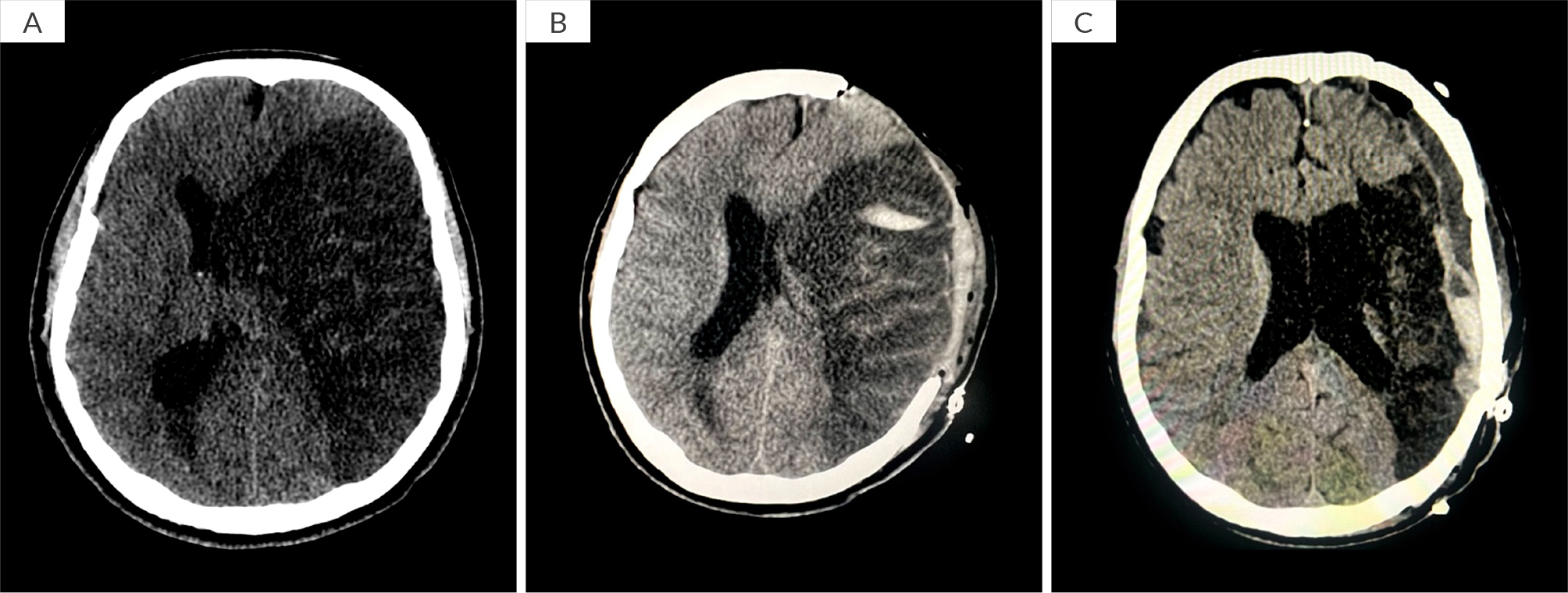
Initially, the patient was haemodynamically stable and his physical examination was normal, except for bilateral coarse crepitations. However, a drop in haemoglobin from 140 g/l to 123 g/l in a span of 7 hours was noted in his routine blood tests, while the other blood tests including white blood cells, platelets, basic metabolic panel (BMP), coagulation panel, troponin and liver function tests were all within normal range. The patient’s sputum culture and pneumonia deoxyribonucleic acid (DNA) panel were both positive for Pseudomonas spp. The patient had recurrent episodes of haemoptysis in the past with usually minimal blood loss; the last episode was 6 months ago with 20–30 ml of blood loss. He had history of bronchiectasis and recalled having chronic lung disease as a young child. He does not recall having a bronchoscopy, chloride sweat test or cystic fibrosis transmembrane conductance regulator (CFTR) gene testing in the past. He has two children (twins) who were conceived via in vitro fertilization. Furthermore, his brother and father also suffer from chronic lung disease. The patient denied any rash, arthralgia, arthritis or upper respiratory infection symptoms. There were no bruises, coagulation disorders or history of anticoagulant use, injury or recent travel. He denied using alcohol or illicit drugs. There was no family history of autoimmune diseases or vasculitis. A chest computer tomography (CT) scan confirmed chronic extensive bronchiectasis, mainly in the lower lobes, as well as bronchial artery shunting (Fig. 1). Despite medical treatment he continued to bleed and was later admitted to the intensive care unit (ICU). He was electively intubated and underwent a bronchoscopy, which showed active bleeding from the right bronchial tree, specifically the bronchus intermedius. Consequently, the patient underwent BAE, targeting the right bronchial artery originating from the aorta, using particle embolisation (300–900 µm). Additional embolisation procedures were performed on branches from the right subclavian, internal mammary artery and phrenic vessels. Post procedure, the patient was deeply sedated (fentanyl and midazolam) for 48 hours. His haemoglobin was stable, and no more bleeding was observed. On day 3, all sedation was discontinued but despite that, his Glasgow coma scale (GCS) did not improve. On day 5, he had a GCS of 5; a full neurological examination revealed unequal pupils, and a positive Babinski sign on the right foot. An urgent head CT scan revealed a large acute infarct in the left middle cerebral artery (MCA) territory with cytotoxic oedema, resulting in a midline shift of approximately 1.5 cm to the right (Fig. 2A). Following decompressive craniectomy procedure (Fig. 2B), he underwent daily neurological assessments and showed gradual improvement over time but remained on a mechanical ventilator with remnant aphasia and right sided weakness due to the affected area of the infarct. A tracheostomy was performed, and the patient underwent an intensive rehabilitation programme.
Figure 1. Imaging features suggest bronchial artery shunting and engorgement due to chronic lung extensive bronchiectasis with lower lobe predominance.
Figure 2. Imaging features suggest bronchial artery shunting and engorgement due to chronic lung extensive bronchiectasis with lower lobe predominance.
Three months post-decompressive craniectomy procedure, the patient was successfully weaned off the mechanical ventilator, and cranioplasty and reposition of the skull bone flap was successfully performed (Fig. 2C). He was discharged home and has demonstrated significant physical and functional improvement. He was able to obey instructions, formulate few sentences, walk with the aid of a cane and feed orally.
DISCUSSION
This case is a rare example of a massive MCA infarction following BAE. Previous reports suggest possible mechanisms that include retrograde flow of embolic material to the subclavian artery or aorta[9], abnormal anastomosis between the bronchial artery and intercostal arteries[6], fistula formation between the bronchial artery and vertebral artery or any other vessels[4], which may occur with long-standing inflammatory lung disease, and right-to-left shunt[8].
In our case we believe it is either a right-to-left shunt from the bronchial artery to the pulmonary vein then into the systemic circulation, or a retrograde flow of the embolic material to the subclavian artery. This is particularly given the complication of massive MCA infarction in our case and extensive BAE procedure in the background of bronchiectasis[10].
To minimise the risk of this serious and possible fatal complication, we categorised the preventive measures to pre-procedure and intra-procedure.
Before BAE, it is recommended that any presence of abnormal vessels or right-to-left shunt is detected, especially in a high-risk patient i.e. with cystic fibrosis or with ongoing chronic inflammatory lung disease. This can be done by an echo with bubble study, or arteriogram and bronchial angiogram. The presence of early pulmonary artery filling would also suggest a right-to-left shunt[4].
It is suggested that visualisation of the anterior spinal artery and proximal subclavian artery on a pre-embolisation study should be considered a contraindication to embolisation at that level[3,6].
During BAE, to minimise the complications stated, using super selective embolisation with a microcatheter, and choosing the right embolic material is of utmost importance. There were 2022 practice guidelines for BAE issued by the European Society of Cardiovascular and Interventional Radiology, which recommend the use of non-spherical polyvinyl alcohol (PVA) particles with a diameter of 300–500 μm, and calibrated spherical microspheres larger than 300 μm as a substitute for PVA particles[10]. The diameter of the anastomotic branch of the bronchial artery is approximately 72–325 μm, and when the diameter of the embolic agent is less than 325 μm, the particles may enter the pulmonary artery through the anastomotic branch and cause non-target vascular embolism[10].
According to one case report[3], if prominent shunting is seen to the pulmonary arterial system, the size of the embolic agent should be increased; this is believed to decrease the risk of non-target embolisation[3]. Several studies have shown the more uniform tris-acryl microspheres (Embosphere®) to be safe and effective for BAE. Reported sizes ranged from 500 to 700 and 700 to 900 μm with high rates of technical success[3].
In another case report it is mentioned that patients who underwent BAE with coils had a lower prevalence of spinal cord infarction than patients who underwent BAE with gelatin sponge particles or n-Butyl-2-cyanoacrylate[5].
After the procedure, the patient should be monitored closely for any abnormal neurological signs or symptoms. This includes delay in recovery after sedation, change in mental status and abnormal neurological signs that need prompt urgent brain imaging.
CONCLUSION
BAE is the mainstay treatment of massive haemoptysis, but it also carries a risk of serious but rare complications. Our discussion covers the possible mechanism of non-target embolisation to the cerebral circulation and the suggested preventive measures to avoid these complications which include pre-procedure imaging to vessels and technical measures. This also emphasises the importance of interventionalist awareness regarding the variant anatomy of bronchial arteries and the possibility of the passage of embolic material. Close monitoring in post-BAE patients for any abnormal neurological signs that warrant urgent brain imaging and early recognition can save patients from a disabling stroke by having the appropriate hyperactive stroke management plan including mechanical thrombectomy.