ABSTRACT
Neutropenia by non-chemotherapy drugs is an extremely rare idiosyncratic life-threatening drug reaction. Ceftriaxone and meropenem are widely used broad-spectrum antibiotics and are generally safe and well tolerated. The authors present a case of neutropenia induced by ceftriaxone and meropenem in an adult patient. The resolution of neutropenia occurred within 48 hours of ceftriaxone and meropenem being discontinued. Although antibiotic-induced neutropenia is uncommon, clinicians should be mindful of this adverse drug effect because of its potential development of severe neutropenia, septicaemia, septic shock, deep-seated infections and even death. Therefore, neutropenic sepsis treatment should be initiated without delay, particularly if the patient becomes septic and febrile. Granulocyte-colony stimulation factor (G-CSF) may be administered to facilitate the recovery process with daily monitoring of neutrophil count. Mortalities from antibiotic-induced neutropenia remain rare, with a range of 2.5–5%.
KEYWORDS
Antibiotic-induced neutropenia, ceftriaxone, meropenem
LEARNING POINTS
- Beta-lactam antibiotics and cabapenem are widely prescribed antibiotics for the treatment of various infections, but they can uncommonly cause neutropenia as adverse effects.
- Severe neutropenia may lead to severe life-threatening sepsis, shock and even death.
- Drug-induced neutropenia typically improves with the cessation of offending agents, supportive treatment and granulocyte-colony stimulating factor (G-CSF) which may shorten the recovery time.
INTRODUCTION
Neutrophils, granules containing white blood cells, constitute a vital component of the primary immune system against various infections[1]. Neutropenia denotes a reduction in the absolute neutrophil count (ANC) below 1.5 × 109/l; severe neutropenia is defined as an ANC below 0.5 x 109/l[1]. Drug-induced neutropenia refers to neutropenia arising as a consequence of pharmacological agent exposure[2]. Although any drugs can potentially elicit this condition, chemotherapy and immune modulator agents are particularly implicated. However, 5–15% of drug-induced acute neutropenia is attributable to antibiotics such as beta-lactam and glycopeptides[3,4].
Ceftriaxone – a third-generation cephalosporin – and meropenem, a carbapenem, are widely used broad-spectrum beta-lactam antibiotics for life-threatening infections in many clinical settings. Ceftriaxone is a first-line empirical antibiotic for bacterial meningitis. Meropenem is often used for severe intracerebral infection due to its good penetration into cerebrospinal fluids and severe pneumonia. Both of these antibiotics are generally safe and well tolerated. Nevertheless, ceftriaxone-induced neutropenia has been reported in previous case studies and trials. Additionally, in our literature research, meropenem-associated neutropenia has been rarely reported in adults but in a few cases, in neonates and infants[5].
The authors present a case of neutropenia induced by ceftriaxone and meropenem. Neutropenia was detected on the 13th day of ceftriaxone and the ANC continued to decrease with meropenem. The number of white cells improved with the cessation of all antibiotics. Although rare, antibiotic-induced neutropenia should be highlighted due to its potential mortality with septicaemia.
CASE DESCRIPTION
A 31-year-old male patient, with no significant past medical history, received treatment for Streptococcus pneumoniae bacterial meningitis during the previous two weeks with intravenous (IV) ceftriaxone 2 g twice daily under the interim care team. However, on the thirteenth day, the patient was re-admitted with an episode of self-resolving low-grade fever and neutropenia of ANC 0.8 × 109/l. He had no other accompanying infective symptoms, neurological manifestations (headache, vision problems, weakness), joint pain, photosensitivity, weight loss or appetite changes, rash or family history of autoimmune diseases. The physical examinations were unremarkable with the absence of meningeal signs and lymphadenopathy.
Considering the potential diagnosis of neutropenic sepsis secondary to overwhelming meningitis and septicaemia, intravenous meropenem was administered initially with daily monitoring of the ANC. Other possible differential diagnoses were viral-induced neutropenia, bone marrow failure, lymphoma and autoimmune disorders.
Further investigations to explore the causes of the neutropenia were conducted. The complete blood count identified moderate neutropenia with mature neutrophils alongside atypical, reactive lymphocytes. No immature cells or blasts were in the blood film. Haemoglobin, reticulocyte count, C-reactive protein, folate, vitamin B12 and iron panel, blood cultures, urine culture, chest X-ray, complement level, rheumatoid factor and autoimmune disorder screening yielded unremarkable results. Negative results were obtained from the influenza swab and COVID-19 PCR, viral serologies (hepatitis, HIV, cytomegalovirus, Epstein-Barr virus). The MRI brain scan showed no significant intracranial abnormality with the resolution of the high signals on diffusion-weighted imaging in bilateral occipital horns compared with the previous 2-week MRI scan.
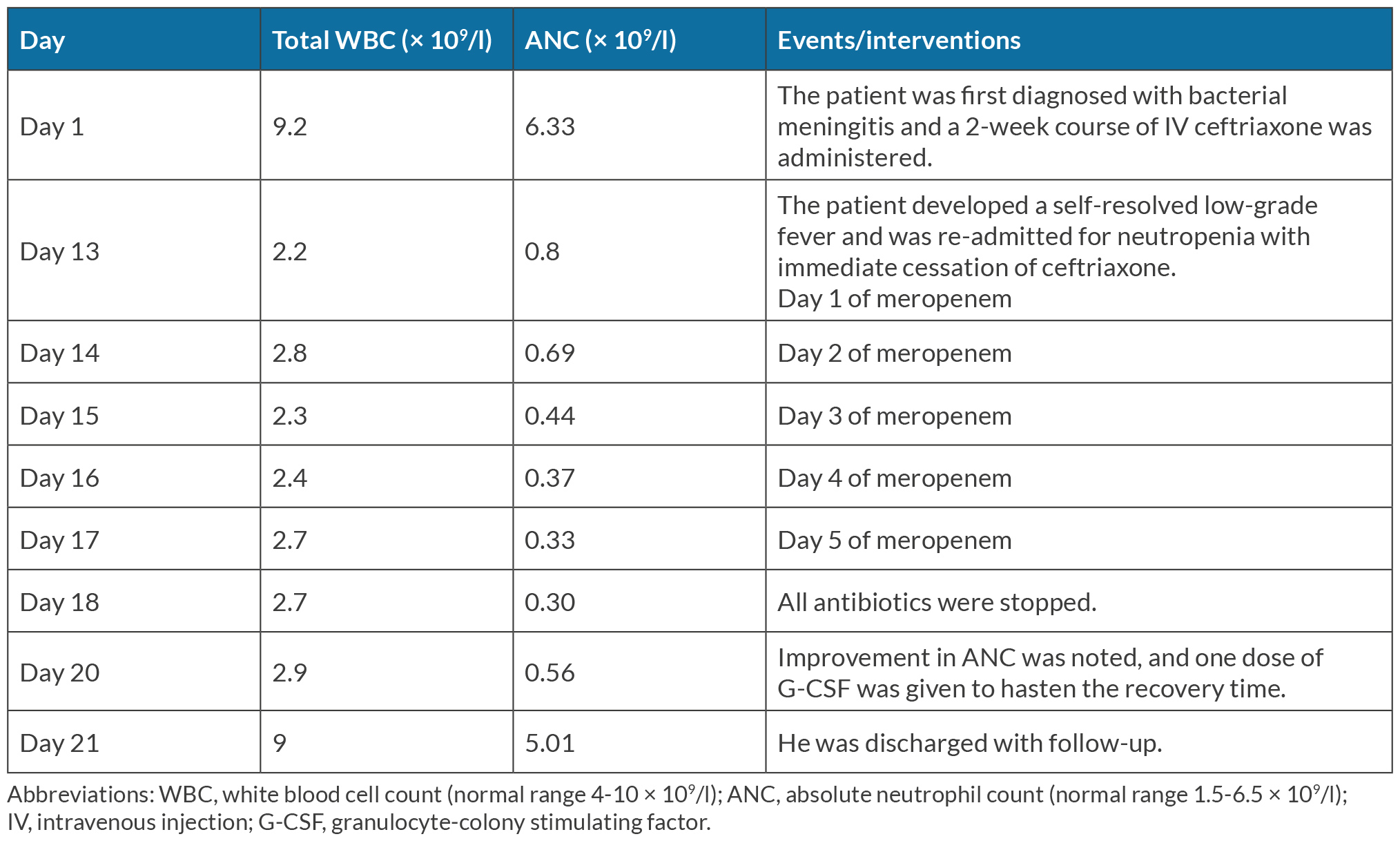
Throughout the admission, the patient remained afebrile and asymptomatic. The investigations effectively excluded infectious aetiologies, rheumatologic disorders and nutritional deficiencies as the underlying causes of neutropenia. This clinical scenario led us to consider antibiotic-induced neutropenia. Despite ceftriaxone cessation for five days, total white cells and the ANC kept decreasing. Consequently, we consider that meropenem might contribute to neutropenia as well. Therefore, we decided to stop all antibiotics after we had discussions with the microbiologist, haematologist and the patient; the ANC improved within 48 hours of antibiotic cessation. A single dose of granulocyte-colony stimulating factor (G-CSF) was administered on the 20th day to expedite the recovery process. Subsequently, the patient was discharged with community-based follow-up. The timeline of the patient is summarised in Table 1.
DISCUSSION
This case report highlights neutropenia induced by ceftriaxone and meropenem in an adult. The pathogenesis of non-chemotherapy drug-induced neutropenia is idiosyncratic and is not yet fully understood. Hypotheses of immune-related mechanisms, direct damage to bone marrow, genetic polymorphism, haptens and drug oxidative modifications are widely accepted[1]. The risk is increased with high-dose or prolonged treatment regimens.
Ceftriaxone-induced neutropenia is regarded as a probable relation in previously published cases. Our patient developed established neutropenia after receiving 4 g of ceftriaxone daily over a two-week period, which aligns with previous reports where neutropenia occurred within a mean time of 5–49 days with the daily dose of 2–4 g of ceftriaxone[3,6]. The average elimination half-life of ceftriaxone in healthy adults is 5.8–8.7 hours[7]. We expected that the ceftriaxone effect should be over after cessation for 5 days (days 13–17). Unfortunately, the ANC continued to decrease with meropenem. Thereafter, we decided to stop all antibiotics as the patient was clinically stable. We noticed an increase in ANC on the 20th day. Therefore, we concluded that both ceftriaxone and meropenem played a role in the neutropenia of our patient.
Neutropenia is a rarely reported adverse effect of meropenem in adults. The World Health Organization Global Individual Case Safety Report database shows a post-marketing surveillance of drugs from the Collaborating Centre for International Drug Monitoring in Uppsala, Sweden. Between 1995 and 2011, severe neutropenia associated with meropenem was reported in 52 cases in a total of 1,959 adverse drug reactions[5].
Non-chemotherapy drug-induced neutropenia typically resolves over time through the identification and immediate cessation of offending medications, along with supportive care and close monitoring. Administration of granulocyte-colony stimulating factor (G-CSF) (filgrastim) can decrease the time of resolution and lower the risk of infectious and fatal complications. Despite no significant reduction of case-fatality rates, the use of G-CSF can shorten the median resolution time to 2.1–3.5 days (range 1–9 days) compared to 7.8 days (range 2–20 days) without G-CSF[1,3,6]. Remarkably, our patient demonstrated rapid and significant improvement in the ANC within 24 hours following a single dose of G-CSF.
The Naranjo score, a commonly used tool to evaluate the probability between an adverse event and drug therapy, was 8 in our patient[8]. This high score indicates a high probability of the adverse effect being caused by ceftriaxone and meropenem; this is supported by the improvement in ANC after discontinuation of antibiotics. It is considered unethical and risky to rechallenge the suspected antibiotics to confirm the causality. However, we recommend close monitoring should ceftriaxone and meropenem be required in the future.
Although the incidence of antibiotic-induced neutropenia is infrequent, it necessitates early recognition, especially in patients receiving prolonged or high-dose therapy. Fatalities related to antibiotic-induced neutropenia remain rare, with a reported range of 2.5–5%[1]. A meta-analysis study discovered that patients recovered from antibiotic-induced neutropenia in 95.2% of cases[3]. Nonetheless, this drug reaction should be highlighted in the context of the potential development of severe neutropenia, septicaemia, septic shock and deep-seated infections. Poor prognostic indicators include age over 65 years, frailty, neutrophil count of below 0.1 cells/mm3, septicaemia, renal failure, heart failure and anticipated prolonged severe neutropenia in bone marrow biopsy[1,3]. In cases of febrile neutropenia, investigations for sepsis and prompt antibiotic initiation are essential. However, if drug-induced neutropenia is suspected, immediate withdrawal of the drug is imperative.