ABSTRACT
Khat is a plant that is commonly used for its stimulating effects and is chewed for its psychoactive properties. It creates feelings of euphoria that are similar to when taking amphetamines. There is an association between khat and liver injury, but the mechanism is not well known. We present three cases of khat-induced liver injury. All cases have elevated IgG and either positive antinuclear antibodies (ANA) or anti-smooth muscle antibody (ASMA); each case has a different course and requires different management. One case improved only by stopping khat, one required a short course of steroids and the last case required treatment such as that for autoimmune hepatitis (AIH).
KEYWORDS
Khat, catha edulis, autoimmune hepatitis
LEARNING POINTS
- This is the first report on different courses and management of khat-induced hepatitis.
- Although khat-induced AIH is rare, early detection and management have a significant effect on disease remission.
- Further studies are needed to evaluate the mechanism of how khat-induced autoimmune hepatitis as it is not well understood.
INTRODUCTION
Khat is a stimulant plant that is commonly used in East Africa and the Middle East for its stimulating effects and is chewed for its psychoactive properties[1]. Khat (Catha edulis) contains various psychoactive compounds including cathinone, cathine and norephedrine[2]. This plant is chewed for several hours and then its juice is swallowed, leading to a stimulant effect[3]. The pleasure from khat chewing is due to the euphoric action of cathinone which is a sympathomimetic amine, with properties that mimic amphetamine[4]. Chronic use of khat is associated with several adverse health effects including liver injury. There are few case reports about khat-induced autoimmune hepatitis (AIH). Healthcare providers need to be aware of the potential link between khat use and AIH in at-risk populations, especially in regions where khat chewing is prevalent.
Case 1
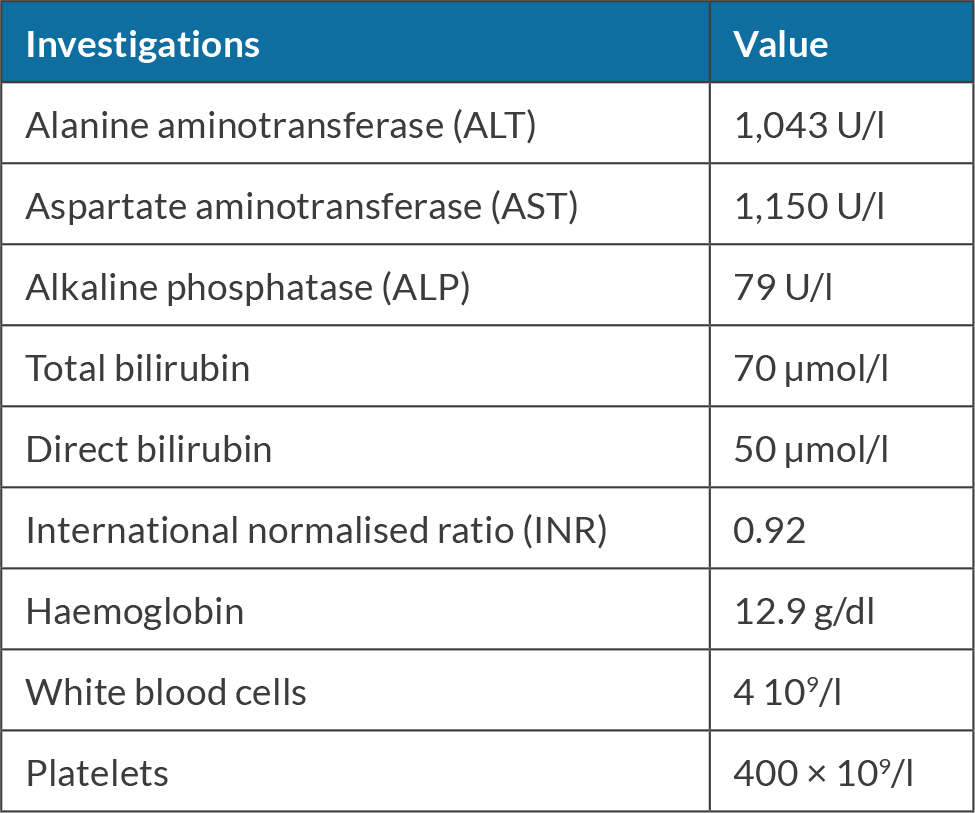
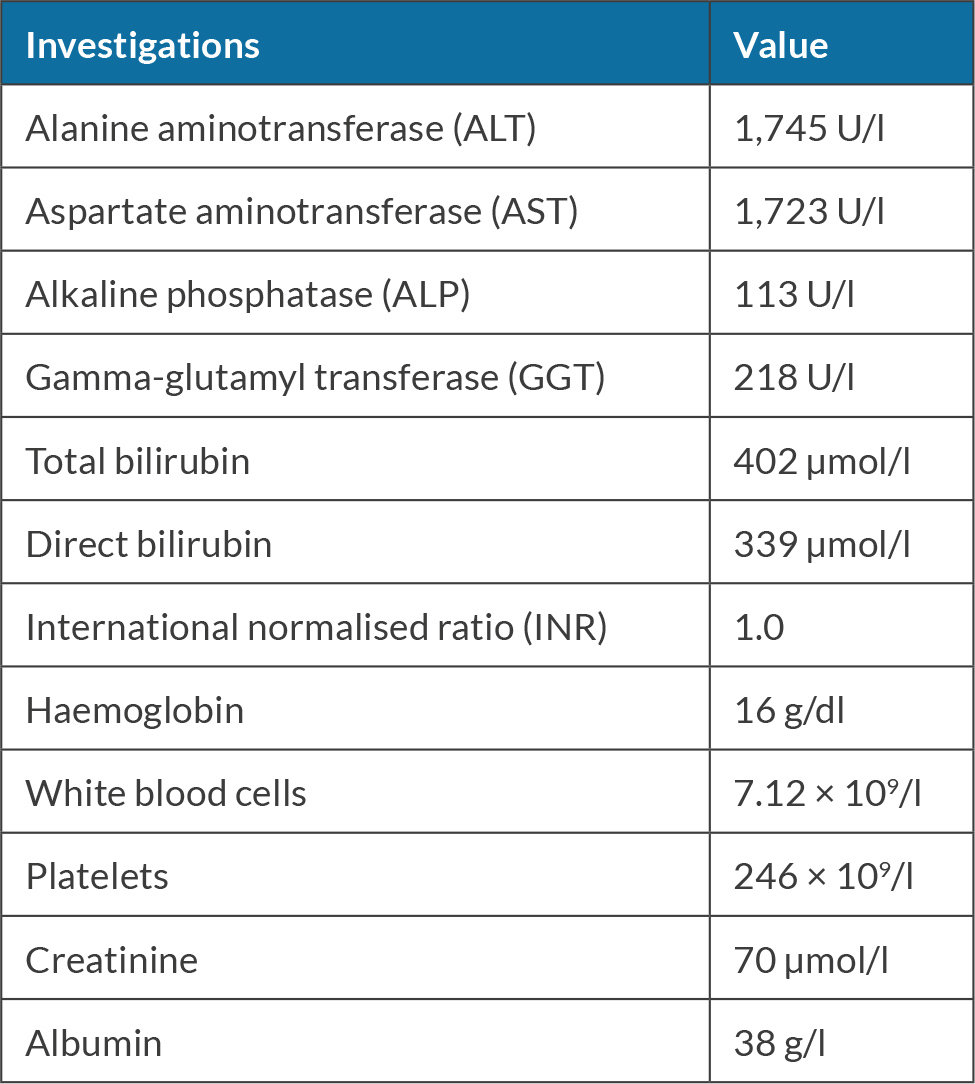
The patient was a 70-year-old female who does not take any medications. She works as a schoolteacher. She has a history of chewing khat almost daily for 10 years; she denies smoking or alcohol consumption. She presented with jaundice and was found to have elevated transaminases of more than 1,000, as shown in Table 1.
On examination, she had jaundice, otherwise examinations were unremarkable. Hepatitis A, B and C serology were negative. She had positive antinuclear antibodies (ANA), negative anti-smooth muscle antibody (ASMA) and elevated IgG of 19 g/l (normal range 7–16 g/l). Laboratory workup did not show any evidence of haemochromatosis, Wilson disease or alpha-1 antitrypsin. An ultrasound scan of the abdomen revealed a normal liver, and patent portal and hepatic veins. The patient was admitted and her liver enzymes and liver function test were monitored for a few days, but her liver enzymes did not improve. She was started on prednisone 40 mg daily. She was discharged after a few days and her aspartate aminotransferase (AST) and alanine aminotransferase (ALT) on discharge were 820 and 723 respectively.
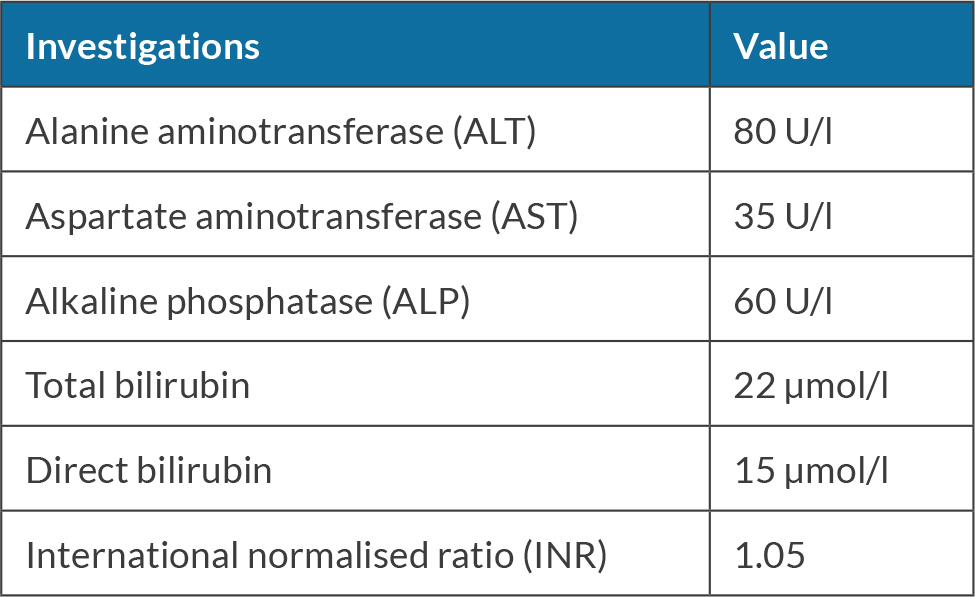
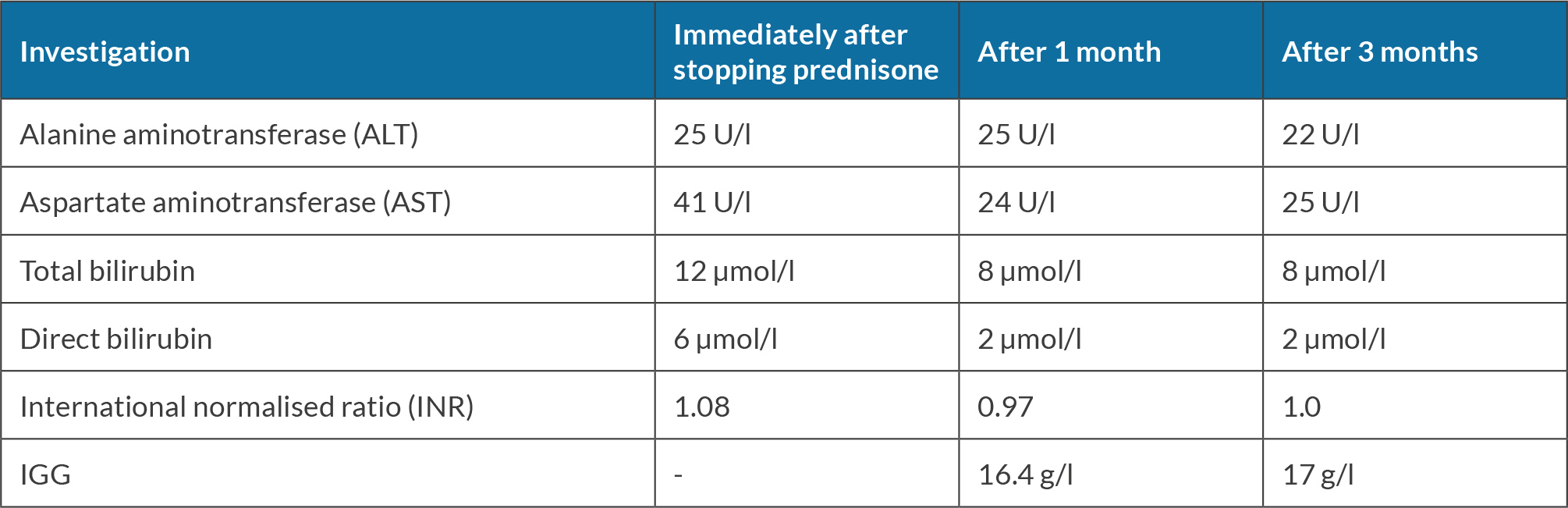
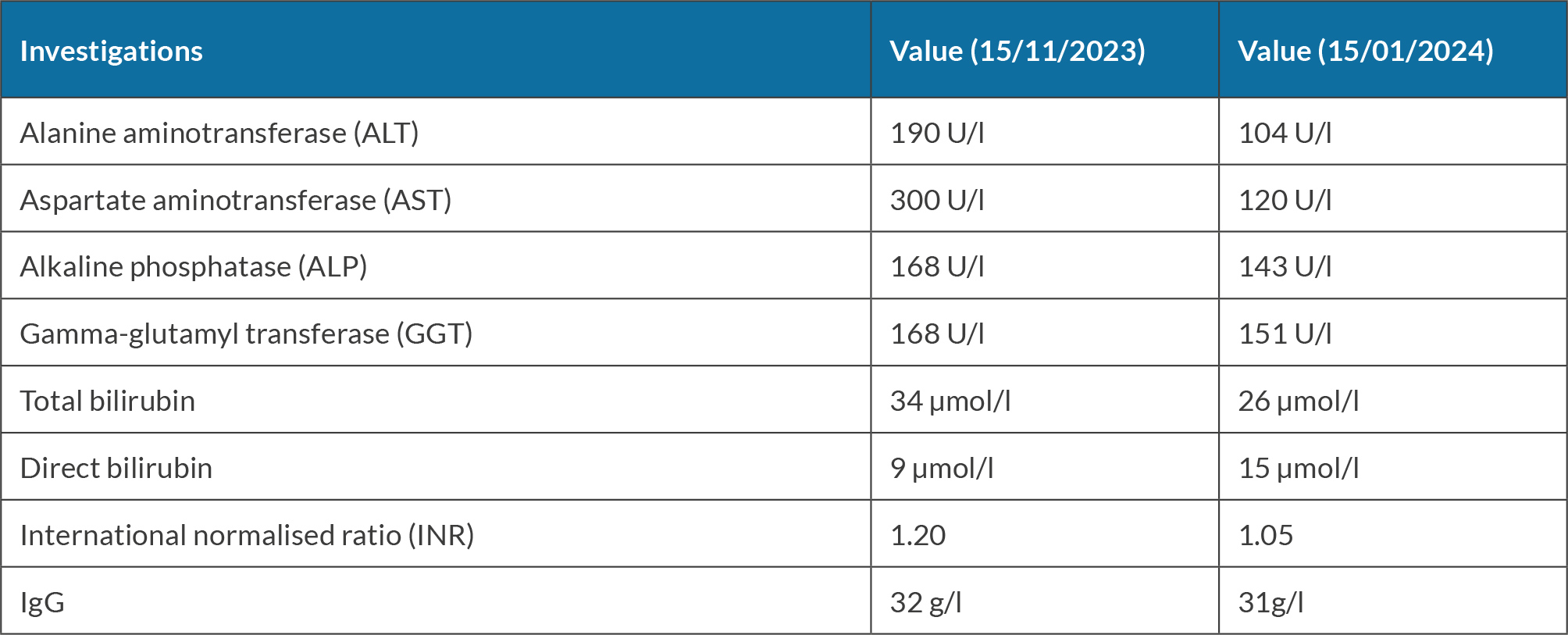
She was seen for a follow-up after one month and she stated that she had stopped chewing khat. Her investigations were repeated (see Table 2). Consequently, prednisone taper was started by lowering the dose by 5 mg weekly until it was stopped. She was seen for follow-up when she was off prednisone and her investigation revealed improvement in liver enzymes and bilirubin, as shown in Table 3.
Case 2
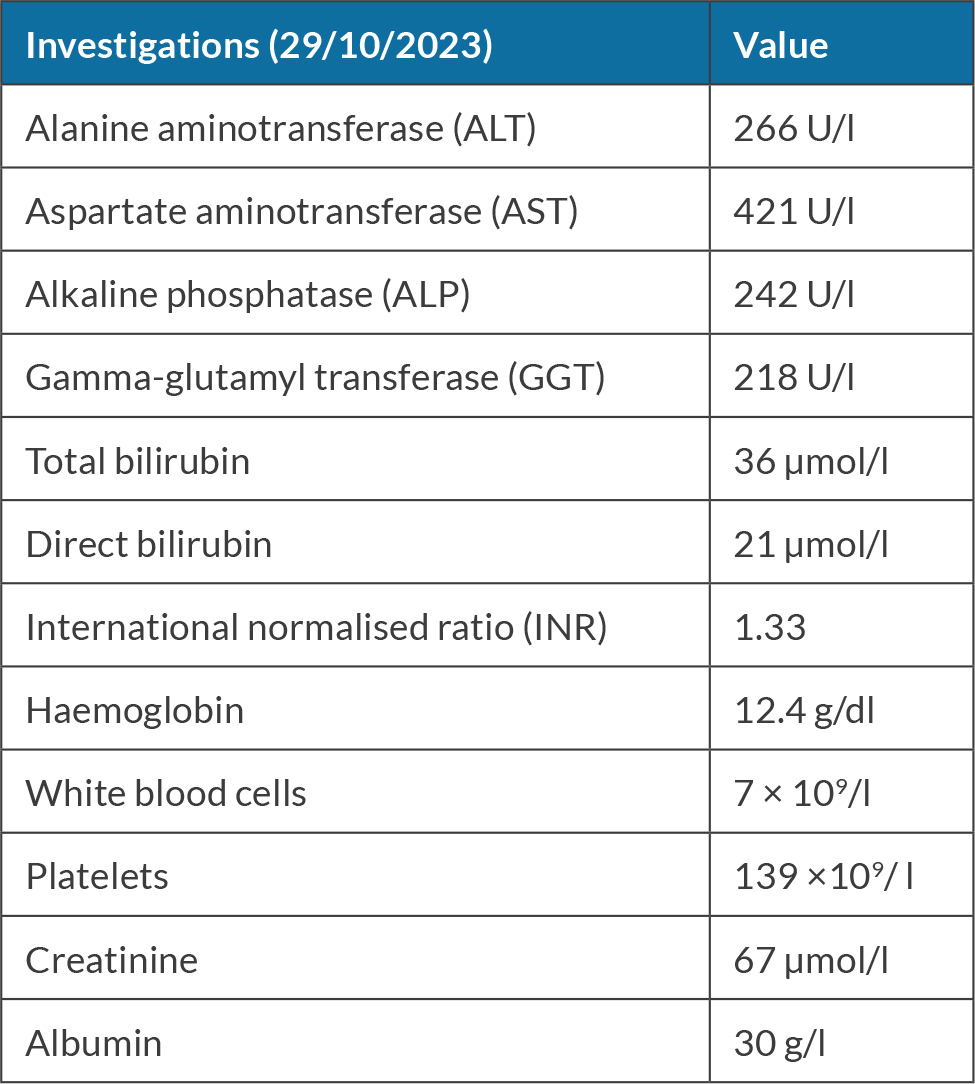
A 41-year-old male presented to the gastroenterology clinic with jaundice. He denied using any prescribed or over-the-counter medications. He had a history of chewing khat almost daily for 15 years. He had smoked 20 cigarettes a day for 10 years and denied alcohol consumption. On examination, he had jaundice, otherwise examinations were normal (see Table 4).
Hepatitis A, B and C serology were negative. He had positive ASMA 1:320, negative ANA and elevated IgG of 37 g/l (normal range 7–16 g/l). Laboratory workup did not show any evidence of haemochromatosis, Wilson disease or alpha-1 antitrypsin. An ultrasound scan of the abdomen revealed a liver of average size with a coarse texture and irregular borders. Normal portal vein calibre measured 11 mm with no evidence of thrombosis.
He stopped chewing khat and was seen for follow-up twice. His liver enzymes trended down without any treatment (Table 5).
Case 3
A 25-year-old male presented with jaundice, nausea and vomiting. He denied using any prescribed or over-the-counter medications. He had a history of chewing khat almost daily for 10 years. He had smoked 20 cigarettes a day for 10 years and denied alcohol consumption. On examination, he had jaundice, otherwise examinations were normal. (Table 6).
Hepatitis A, B and C serology were negative. He had positive ASMA 1:160, positive ANA 1:80 and elevated IgG of 17.4 g/l (normal range 7–16 g/l). Laboratory workup did not show any evidence of haemochromatosis, Wilson disease or alpha-1 antitrypsin. An ultrasound scan of the abdomen revealed normal liver size, and patent portal and hepatic veins. Subsequently, he underwent a liver biopsy which revealed chronic severe active hepatitis with severe portal, interface and lobular inflammation rich in plasma cells infiltrate with severe hepatocellular injury and areas of parenchymal dropout. There was evidence of portal fibrosis with few septa (F2).
He was started on prednisone 40 mg for 30 days then tapered by 5 mg weekly and discharged home. He was advised to stop chewing khat and was booked for follow-up in 30 days. Unfortunately, he did not show up for the follow-up. Then he was seen in the clinic three months later after he completed the prednisone taper. He stated that he had stopped chewing khat, but his liver AST and ALT were more than 1,000 again. He was restarted on prednisone taper and was seen again after 30 days. His investigation revealed AST and ALT of 600 and 160 respectively, and bilirubin of 56, so prednisone was tapered to 10 mg daily and azathioprine 100 mg was added.
DISCUSSION
Khat comes from the evergreen shrub Catha edulis, which is indigenous to various East African countries. It creates feelings of euphoria that are similar to when taking amphetamines. The primary active compound found in khat is cathinone, which is broken down into a less powerful substance called R, S-(-)-norephedrine. Khat leaves are typically consumed soon after harvesting because cathinone loses its potency due to dimerisation occurring a few days after being picked[5].
Khat is a socially accepted drug in Yemen, Somalia, Somaliland, Kenya, Djibouti and Ethiopia, especially in men. In a study around 34% of immigrant Somali people in the UK use khat[6,7]. In another study in Ethiopia relating to Ethiopian college students, around 41% of students had used khat once in a lifetime, usually in conjunction with alcohol[7,8].
The exact mechanism of khat-induced liver injury is still not well known, although animal studies demonstrate fibrosis and liver injury with chronic khat use[9]. It could be by triggering AIH in a genetically susceptible individual, or it could be due to toxic injury related to khat, as khat alkaloids are extensively metabolised by the enzyme cytochrome P450 2D6 in the liver. Accumulation of these metabolites may play a role in liver injury and progression to chronic liver disease[4,10]. It could be due to the liberal use of pesticides or other contaminants during farming that might contribute to the hepatotoxic effect of khat[11]. A study revealed an association between chewing khat and developing chronic liver disease, but in this study no risk factors for liver injury were identified in 53% of the cases with chronic liver disease despite extensive investigations, except that more than 80% of them used khat[10]. Khat-induced AIH can mimic AIH in terms of laboratory findings and histology, making it challenging to differentiate between the two. The injury is typically hepatocellular, causing high transaminase enzymes and in some cases inducing AIH where autoantibodies develop[12].
Treatment typically involves stopping the patient from using khat and monitoring liver enzymes closely, but the pathophysiology of khat-induced liver injury is not well understood. In some cases, corticosteroids and immunosuppressants need to be started. In one of our cases, the patient’s transaminases re-elevated when we tried to taper down steroids, so he was started on steroids in conjunction with azathioprine. Since the pathophysiology and treatment of khat-induced AIH are still not clear, further studies are needed to help gain more understanding of khat-induced AIH.
CONCLUSION
Khat has a significant association with hepatotoxicity and can induce AIH, which mimics AIH in terms of autoantibodies and histology. Management of khat-induced AIH involves cessation of using khat but some cases require immunosuppressant medications.