ABSTRACT
Romiplostim and eltrombopag are synthetic agonists of the thrombopoietin receptor (TPO-R), commonly used for immune thrombocytopenic purpura (ITP) and sometimes in myelodysplastic syndrome (MDS). They are rarely associated with kidney injury. We report a case of acute kidney injury caused by romiplostim and eltrombopag in an 80-year-old male patient with MDS and ITP. He did not have systemic haemolysis syndrome but isolated acute renal thrombotic microangiopathy confirmed by kidney biopsy. He was treated with steroids, plasmapheresis and anticoagulation, with improvement in renal function. Interestingly, the patient had high antiphospholipid (aPL) antibodies noted upon screening, indicating a possible new antiphospholipid syndrome (APS) diagnosis. In the presence of circulating aPL antibodies, eltrombopag may have served as a trigger, causing endothelial injury and subsequent renal microangiopathy; aPL antibodies were still significantly positive at four weeks of outpatient testing. This case and a few others reported in the literature highlight the importance of screening for aPL antibodies before initiating TPO-R agonists in patients with ITP. We suspect that using TPO-R agonists, rather than underlying aPL, caused renal failure.
KEYWORDS
Acute renal failure, acute thrombotic microangiopathy, antiphospholipid syndrome
LEARNING POINTS
- Synthetic agonists of the thrombopoietin receptor, such as romiplostim or eltrombopag, can cause acute renal failure.
- Preexisting antiphospholipid (aPL) antibodies may increase the risk of renal failure.
- Screening for aPL antibodies should be considered before initiating thrombopoietin-receptor agonists (TPO-R agonists) in patients with immune thrombocytopenic purpura (ITP).
INTRODUCTION
Thrombotic microangiopathy (TMA), a pathological lesion observed in various diseases, is triggered by endothelial injury and dysfunction. Although TMA lesions are often accompanied by clinical features of microangiopathic haemolytic anaemia (MAHA), thrombocytopenia and ischaemic end-organ injury, renal-limited TMA is a rare diagnosis requiring a high level of suspicion and quite often leads to delay in the initiation of targeted therapy[1]. Romiplostim and eltrombopag are synthetic thrombopoietin-receptor agonists commonly used for immune thrombocytopenic purpura (ITP) and sometimes in myelodysplastic syndrome (MDS) patients; eltrombopag appears to be highly specific for the TPO-R. There are a few cases of renal toxicity related to romiplostim and eltrombopag reported in the literature. Potential mechanisms of nephrotoxicity are not clear. Interestingly, many patients with immune thrombocytopenia have aPL antibodies and are at risk for thrombosis[2].
CASE DESCRIPTION
Our patient was an 80-year-old man with an eighteen-month history of MDS with multilineage dysplasia, poorly responsive to steroids, with baseline platelet counts between 30,000 and 80,000 cells/mm3. He was first seen for epistaxis symptoms and found to have an undetectable platelet count. ITP was suspected due to profound thrombocytopenia. He was treated with two doses of intravenous immune globulin 1 g/kg and was started on prednisone 1 mg/kg. He also received a platelet transfusion for epistaxis. Two days later, the platelet count was 47,000 cells/mm3 and increased to 116 cells/mm3 in another three days without platelet transfusions, further supporting immune thrombocytopenia. The response was transient, with platelet count falling below 30,000 cells/mm3 despite steroids in the following few days. The steroids were stopped, and he was started on romiplostim. He received five weekly doses (up to 3 mcg/kg) before insurance authorisation for eltrombopag was finally obtained.
Five days after starting eltrombopag 50 mg daily, the patient was seen at the clinic for a routine visit. He complained of generalised weakness and confusion. Bloodwork showed acute kidney injury with creatinine at 2.2 mg/dl and glomerular filtration rate (GFR) of 28 ml/min). One week prior, creatinine had been 1.3 mg/dl (GFR 56 ml/min). He was admitted to the hospital. A computed tomography (CT) scan of the head was negative, and his platelets were at 35,000 cells/mm3. On examination, he appeared euvolemic and renal function continued to worsen, with creatinine increasing to 2.5 mg/dl despite IV fluids. Renal ultrasound showed no acute findings. He had not been on any nephrotoxic medications. A haemolysis workup showed stable haemoglobin, normal haptoglobin and normal bilirubin. ADAMTS13 activity was 55%, and the peripheral smear showed rare schistocytes. Trace proteinuria (189 mg/g) was noted. Serological testing for antinuclear antibodies, antineutrophil cytoplasmic antibodies, and serum and urine monoclonal proteins were unremarkable. Both complements C3 and C4 were low.
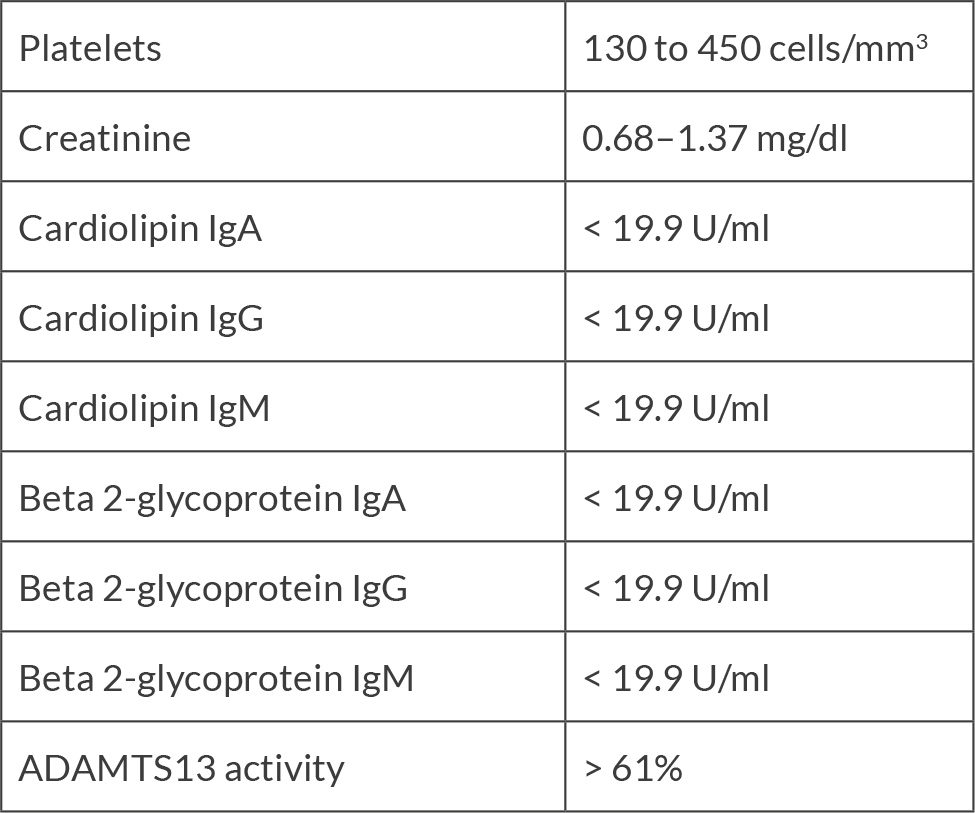
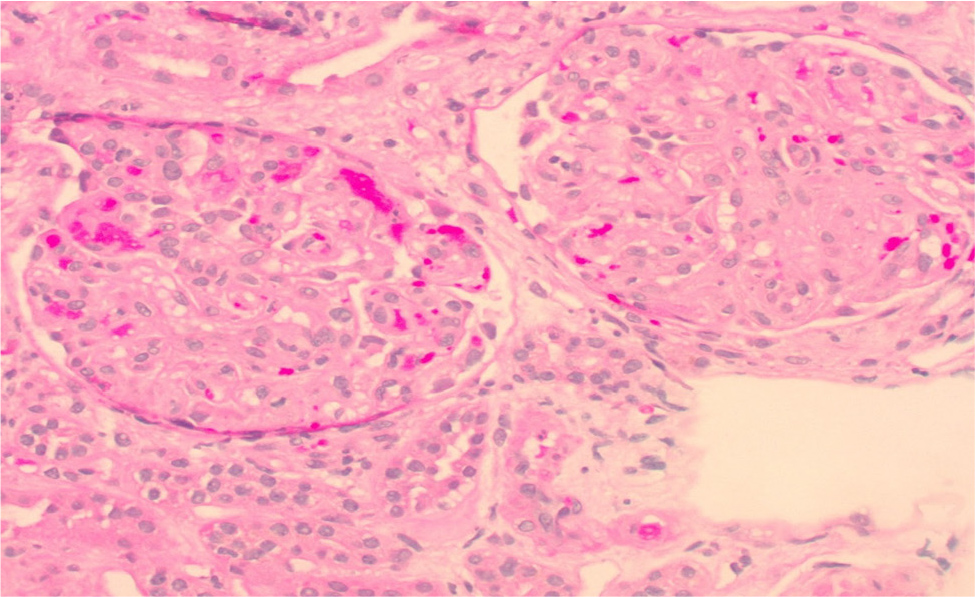
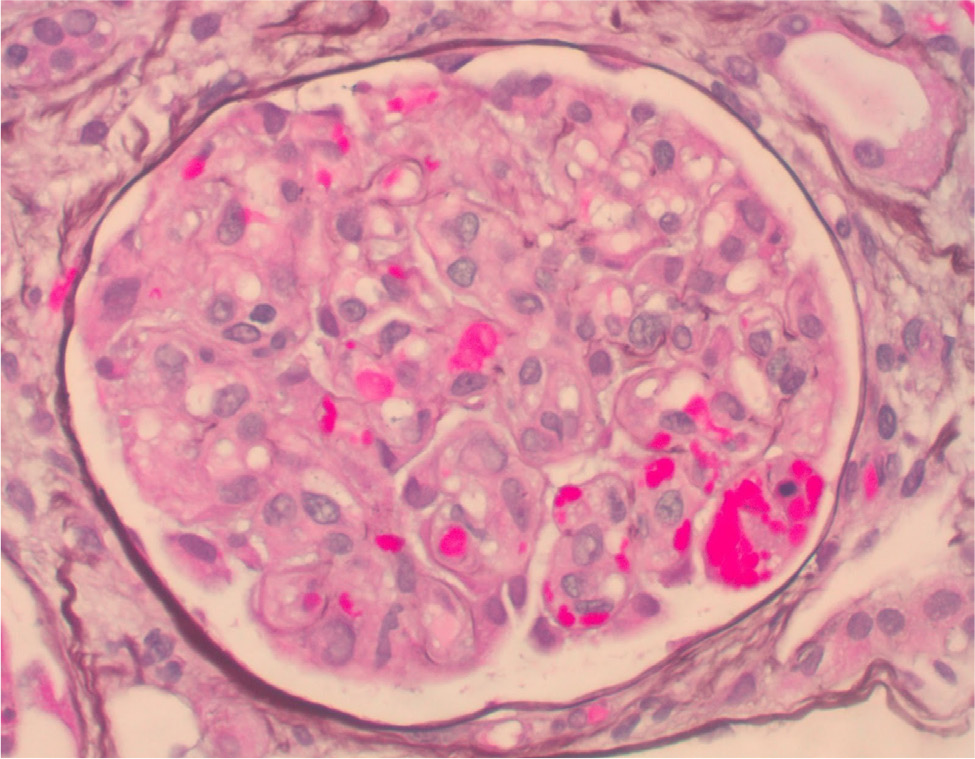
Considering previous case reports of renal failure from eltrombopag use in patients with lupus and aPL, a laboratory screening for this was requested. Lupus anticoagulant was positive by dilute Russell viper venom time (dRVVT) (normalised ratio of 2.09). Cardiolipin IgG was >112, IgM was 63.1 and IgA was >65. Beta 2-glycoprotein IgG was >112, IgM was 56.7 and IgA was >65 (Table 1). The patient underwent five plasmapheresis sessions, and a kidney biopsy was obtained. Renal biopsy showed acute thrombotic microangiopathy with severe endothelial injury, fibrin thrombi in the glomerular capillary loops and an evolving element of chronic thrombotic microangiopathy (Fig. 1 and 2). The patient was started on prednisone 1 mg/kg daily and enoxaparin 1 mg/kg twice daily. Creatinine improved to 1.97 at discharge and 1.45 (GFR ml/min) two weeks later. Repeat cardiolipin and beta-2 glycoprotein IgG and IgA antibodies remained remarkably high, but IgM normalised.
Figure 2. Kidney biopsy: he glomerulus shows fibrin thrombi and schistocytes, and the splitting of the glomerular basement membranes and mesangial expansion.
DISCUSSION
TMA describes a pathological state where vessels are occluded by platelet-rich thrombi, leading to thrombocytopenia and MAHA. Depending upon the cause of the TMA, thrombi can be systemic or intrarenal, and inevitably lead to end-organ damage. Endothelial injury associated with platelet activation and consumption contributes to microvascular thrombosis, tissue ischaemia and subsequent end-organ injury[3]. As red blood cells traverse the microvasculature laden with thrombosis, they become fragmented, producing the hallmarks of MAHA, including schistocytes and thrombocytopenia, with low haptoglobin and elevated indirect bilirubin. Haematuria, proteinuria and hypertension are common with renal involvement[1]. TMA syndromes are extraordinarily diverse. Nine TMA syndromes – including hereditary and acquired – are described in the literature, but we will focus on drug-mediated TMA relevant to our case. Drug-mediated TMA can be from toxic dose-related or immune reactions[4].
APS is an autoimmune condition that greatly increases the risk of arterial and venous thrombosis and pregnancy complications. This increased risk is mediated at least in part by aPL antibodies, which are most effectively screened for with a functional assay known as the ‘lupus anticoagulant’[2]. Although a positive lupus anticoagulant almost certainly portends more risk than other positive tests, it is susceptible to confounders, such as concomitant anticoagulation. Therefore, it must be interpreted cautiously, especially in hospitalised patients. Although the pathophysiology of underlying thrombocytopenia in APS has yet to be definitively revealed, mechanisms that play a role (at least in subsets of patients) include ITP-like autoantibodies against platelet glycoproteins, aPL-mediated platelet activation and consumption, and potentially life-threatening thrombotic microangiopathy. Thrombocytopenia is common in APS and is associated with more severe disease. It occurs in 20–40% of patients with APS, while renal dysfunction develops in ∼25% of patients with primary APS[5]. Corticosteroids, intravenous immunoglobulin, dapsone and rituximab have all been effective in APS patients with thrombocytopenia[6]. Romiplostim and eltrombopag are synthetic agonists of the thrombopoietin receptor commonly used in the US since 2009 for ITP and sometimes in MDS patients[7]. Thrombopoietin-receptor agonists increase platelet count by stimulating megakaryocytes. Eltrombopag is often used in patients with ITP who fail to respond to steroids and immunoglobulin[2].
Our patient presented with acute renal failure within a few months of the use of romiplostim and five days of eltrombopag administration. He had no previous history of thrombotic events that may have indicated a hypercoagulable state, including underlying aPL. Testing for phospholipase antibodies was specifically ordered after a literature review that indicated a correlation[3]. The literature review revealed a few reported cases of acute renal failure associated with eltrombopag[3,7-11]. Sperati et al. describe acute renal failure from eltrombopag in a patient with preexisting APS[3]; Garra et al. mention two other cases of catastrophic antiphospholipid syndrome in lupus patients treated with eltrombopag[10]. Three others noted that the patients had tested negative for aPL antibodies[8,9,11]. Tomov describes a 19-year-old girl with acute kidney injury after romiplostim use for lupus-associated ITP, who underwent a kidney biopsy that revealed thrombotic microangiopathy[11]. She tested negative for aPL. The other two reports[7,8] did not mention testing the patients for aPL. Two of these patients had significant proteinuria and nephrotic syndrome with the use of eltrombopag[12,13].
The ASPIRE study on eltrombopag revealed one case of acute kidney injury along with other common side effects[14]. Garra et al., in a systematic literature review, concluded that TPO-R agonists are associated with thrombotic risk in lupus or APS patients, and that eltrombopag use for such patients may be associated with a potentially devastating adverse thrombotic event or catastrophic antiphospholipid syndrome, independent of the platelet count, time from initial administration and dose[10]. These patients would require close monitoring of kidney function. Potential mechanisms of nephrotoxicity remain speculative. In our case, the Naranjo adverse drug reaction (ADR) scale revealed a score of 7, indicating probable ADR[15]. This scale has been applied in case reports to evaluate the probability of an adverse event caused by an oral drug or therapeutic modality. Other case reports quoted above also reported scores ranging from 5 to 7.
In the 2023 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for APS, thrombocytopenia is included[16]. The occurrence of thrombocytopenia in aPL/APS patients is important because it could predict APS-related clinical events with a three-fold increased risk for thrombotic events, obstetric morbidity or all-cause deaths. In patients with aPL/APS-related thrombocytopenia, whether anticoagulation and anti-aggregation could be a reasonable option for primary thromboprophylaxis remains a research question[16].
CONCLUSION
This case raises concerns about eltrombopag triggering thrombotic microangiopathy in patients with aPL antibodies. Healthcare providers should be alert to the possibility of worsening kidney function in patients with or without underlying APS or lupus, and ITP on thrombopoietin-receptor agonists. Screening for aPL antibodies should be considered before initiating thrombopoietin-receptor agonists in patients with ITP.