ABSTRACT
Background: A case of bilateral multifocal serous retinal detachments and dry eye complicated with unilateral peripheral ulcerative keratitis (PUK) during erdafitinib therapy is described.
Case description: A 76-year-old male underwent a baseline examination two months after initiating 8 mg erdafitinib therapy (April 2023) due to metastatic urothelial carcinoma. Left subfoveal serous retinal detachment was observed initially but the treatment was resumed as he was asymptomatic. In May 2023, bilateral multifocal subretinal fluid pockets were identified, and the patient was still asymptomatic. However, in June 2023 he complained of bilateral redness and a stinging sensation in his right eye. Bilateral severe dry eye and right PUK were diagnosed. He was prescribed dexamethasone eye drops and sodium hyaluronate artificial tears for both eyes. One week later corneal staining decreased, and progression of PUK ceased. Erdafitinib therapy was discontinued in June 2023 due to the planned transurethral prostatectomy. By July 2023, after discontinuation of the drug and administration of the topical treatment, the dry eye improved and the PUK became inactive. There was also resolution of subretinal fluid pockets in the right eye and a reduction of subretinal fluid pockets in the left eye. After the reinitiation of erdafitinib therapy, serous retinal detachments recurred in both eyes in September 2023, but both corneas remained stable with topical low-dose dexamethasone, cyclosporine-A and artificial tear usage.
Conclusion: Erdafitinib therapy may lead to concurrent anterior and posterior segment complications. Multidisciplinary monitoring is crucial for patients undergoing erdafitinib therapy to prevent possible visual disturbances.
KEYWORDS
Erdafitinib, peripheral ulcerative keratitis, serous retinal detachment
LEARNING POINTS
- Erdafitinib, a tyrosine kinase inhibitor of fibroblast growth factor receptors 1 to 4, is administered for the treatment of locally advanced, unresectable or metastatic urothelial carcinoma but however is fraught with several systemic and ocular side effects.
- Concurrent anterior and posterior segment ocular involvement could be encountered in patients undergoing erdafitinib therapy.
- Maintaining a high level of suspicion and closely monitoring for potential ocular complications through collaborative efforts is essential for all patients undergoing erdafitinib therapy.
INTRODUCTION
Remarkable advancements have been achieved in the field of cancer treatment, leading to the development of targeted therapeutic drugs. These treatments help clinicians to specifically impede certain mutations, selectively affecting the tumour cells while minimising the harm to healthy tissues, offering fewer adverse effects compared to the traditional chemotherapeutics[1,2].
Fibroblast growth factor receptor (FGFR), located on the cell membrane, is one of the targeted molecules for the treatment of various cancers; 7.1% of all cancer types are reported to have a molecular aberration involving this pathway[1]. Erdafitinib, a tyrosine kinase inhibitor of FGFRs 1-to-4 (pan-FGFR inhibitor), was approved by the US Food and Drug Administration as a first FGFR kinase inhibitor for the treatment of locally advanced, unresectable or metastatic urothelial carcinoma. This is where FGFR pathway alterations have been identified up to 20% of the cases and, follows the BLC2001 trial in April 2019[2,3]. Since then, several systemic adverse effects have been reported in association with erdafitinib such as hyponatraemia, stomatitis, asthenia, hyperphosphataemia, nail disorders, skin disorders and diarrhoea[2]. Ocular adverse effects such as dry eye[3], conjunctivitis[3], corneal thinning and ulceration[4], acute-onset white cataract[4], serous neurosensory retinal detachment[5] and subretinal pseudo-vitelliform accumulation[6] have also been reported.
We report a case with metastatic urothelial carcinoma where the patient presented with bilateral asymptomatic multifocal serous retinal detachments and unilateral peripheral ulcerative keratitis (PUK) during the erdafitinib therapy, which were partially resolved after the cessation of the drug.
CASE DESCRIPTION
A 76-year-old actively working surgeon underwent a baseline ocular examination in April 2023, as his oncologist advised him to be under scrutiny for possible eye complications. He was on 8 mg of peroral erdafitinib treatment for two months due to metastatic urothelial carcinoma. He was first diagnosed to have a high-grade invasive papillary urothelial carcinoma in January 2022. A positron emission tomography (PET) scan had showed pathological fluorodeoxyglucose (F-18 FDG) uptake in bilateral retrocrural, para-aortic, interaortocaval and left common iliac lymph nodes. He had metastatic upper urinary urothelial carcinoma and was administered four courses of cisplatinum plus gemcitabine and pembrolizumab systemic therapies. He underwent right radical nephrectomy and lymph nodes adenectomy surgery in May 2022. A pathologic examination revealed a renal high-grade invasive papillary urothelial carcinoma. After surgery, he received four more cycles of cisplatinum plus gemcitabine and pembrolizumab systemic therapies and three cycles of nivolumab monotherapy. In October 2022 a PET/computerised tomography scan showed increased F-18 FDG uptake in bilateral retrocrural, interaortocaval, left common iliac, left external iliac and left obturator regions lymph nodes, and the patient received radiotherapy. After radiotherapy, liver metastases were detected in February 2023. As the FGFR test was positive in tumour specimen, peroral erdafitinib treatment (Balversa®, Janssen Pharmaceutica) was started in February 2023.
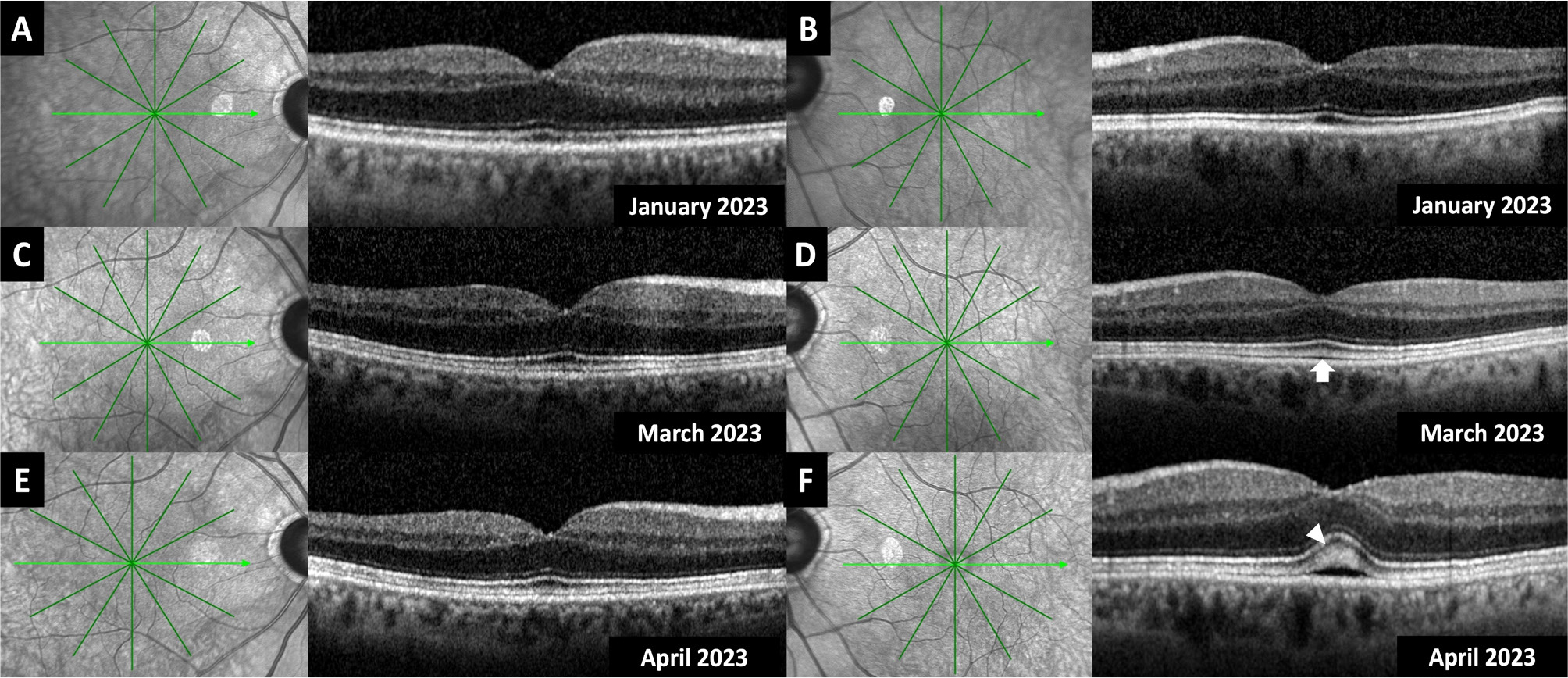
During this time frame, he also underwent sequential uneventful bilateral cataract surgery for grade-4 nuclear sclerosis in January 2023, one month before the initiation of erdafitinib. He had routine macular spectral-domain optical coherence tomography (SD-OCT) (Heidelberg Spectralis®, Heidelberg Engineering) prior to January 2023 (Fig. 1A, B) and two months after the cataract surgeries (March 2023) (Fig. 1C, D).
Figure 1. Transfoveal spectral-domain optical coherence tomographic sections of both eyes one month before (January 2023) (A, B), one month after (March 2023) (C, D) the uneventful cataract surgeries and two months after the initiation of erdafitinib therapy (April 2023) (E, F). The white arrow indicates the thickening of the ellipsoid and interdigitation zones and the white arrowhead indicates the accompanying subretinal fluid.
In April 2023, he was examined again as his oncologist demanded an eye examination related to 8 mg erdafitinib usage. He was visually asymptomatic. Best-corrected visual acuity (BCVA) was 7/10 on a Snellen chart in OD (oculus dexter – right eye) with the correction of −1.25 x 120 (cylinder × axis), and 6/10 in OS (oculus sinister – left eye) with the correction of −1.25 × 70. Slit-lamp examination was unremarkable. Intraocular pressures were within normal limits OU (oculus uterque – both eyes). Both eyes looked normal ophthalmoscopically. However, while the spectral-domain optical coherence tomography (SD-OCT) sections passing through the fovea was normal in OD (Fig. 1E), there was subfoveal neurosensory detachment with accompanying subretinal fluid and thickening of the ellipsoid and interdigitation zones in OS (Fig. 1F). We concluded that erdafitinib-induced retinopathy was present in OS. As he was asymptomatic, the medication was not stopped.
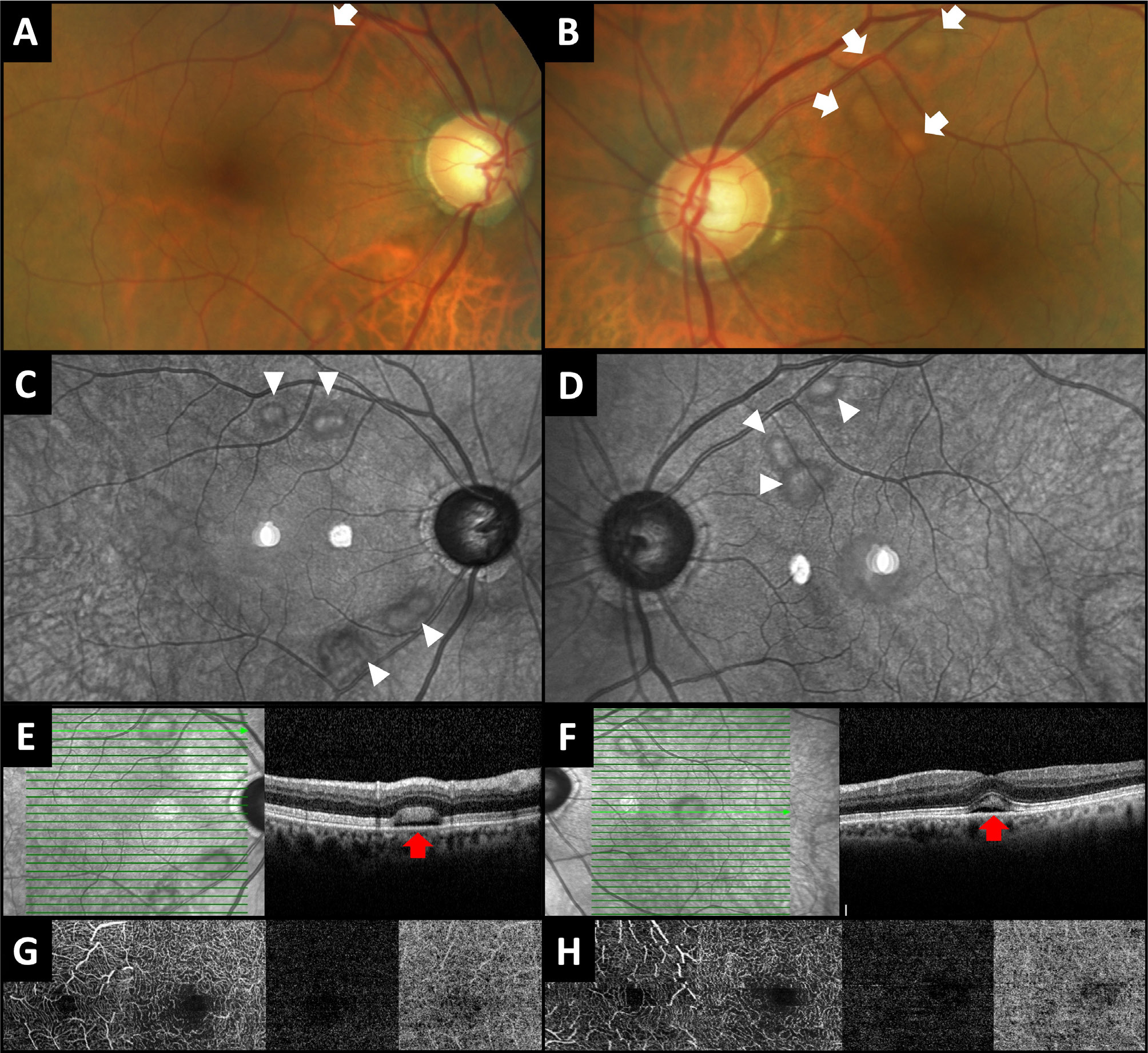
In May 2023, his BCVA remained stable and both anterior segments were normal. However, bilateral, multifocal subretinal fluid pockets were observed around the temporal vascular arcades on fundoscopy (Fig. 2A, B). Infrared images (Heidelberg Spectralis, Heidelberg Engineering) also showed bilateral, multifocal hyper-reflective spots encircled by hypo-reflective areas (Fig. 2C, D). The SD-OCT sections passing through the pockets revealed the presence of neurosensory retinal detachment with subretinal fluid and thickening of the ellipsoid and interdigitation zones bilaterally (Fig. 2E, F). OCT-angiography (OCT-A) (Triton, Topcon Inc.) 3×3 mm sections centred at the fovea depicted no remarkable findings on superficial and deep capillary plexuses, outer retina and choriocapillaris slabs in either eye (Fig. 2G, H).
Figure 2. May 2023. Colour fundus pictures (A, B) depicting the subretinal fluid pockets as round yellowish lesions (white arrows). Multifocal hyper-reflective spots encircled by hypo-reflective areas (white arrowheads) were noticed on infrared images (C, D) corresponding to the pockets. Spectral-domain optical coherence tomographic sections passing through the pockets (E, F) revealed thickening of the ellipsoid and interdigitation zones together with subretinal fluid (red arrows). No remarkable findings were observed at the superficial capillary plexus, deep capillary plexus, outer retina and choriocapillaris slabs of 3×3 macular optical coherence tomography angiography sections (G, H).
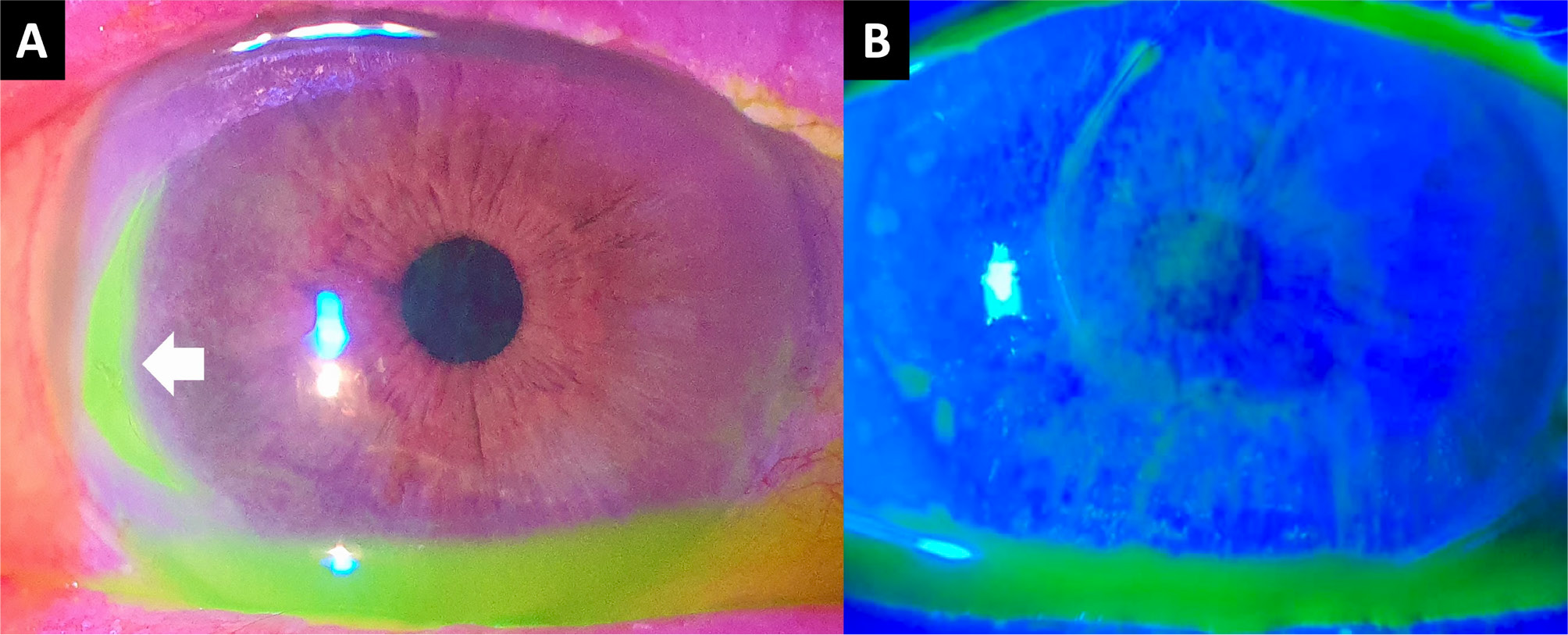
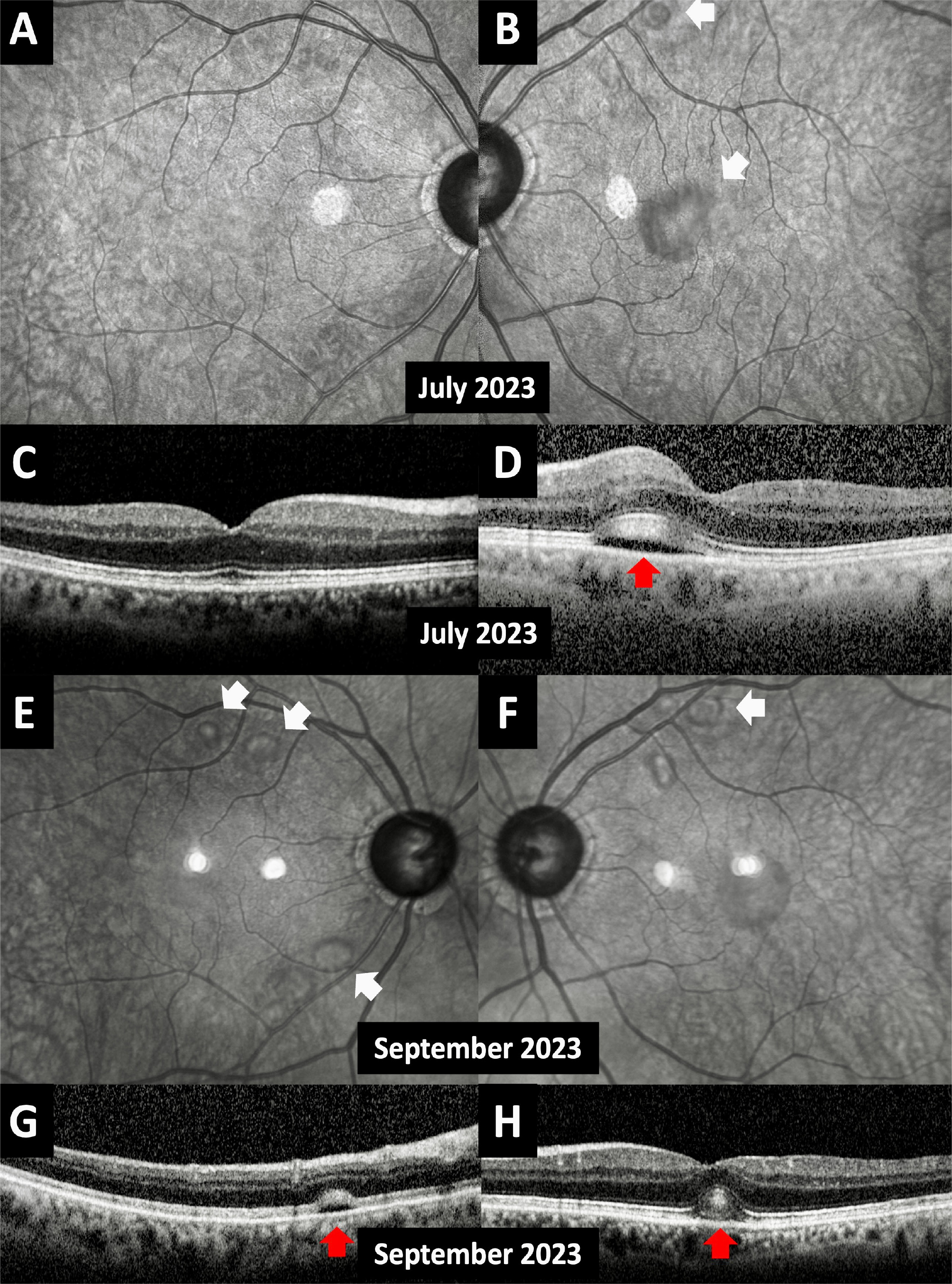
The following month, June 2023, the patient complained of bilateral redness and a stinging sensation. The erdafitinib dosage was reduced to 7 mg three weeks before this visit. On slit-lamp examination, bilateral severe dry eye and right PUK were diagnosed (Fig. 3). The patient was prescribed dexamethasone 0.2% eye drops four times a day and sodium hyaluronate artificial tears hourly for both eyes. Meanwhile, as a transurethral prostatectomy was planned, erdafitinib therapy was halted. Marked improvement of PUK, dry eye and bilateral macular serous detachment was observed one week after the discontinuation of the drug. In July 2023, a corneal ulcer healed with minimal stromal scarring in OD and dry eye symptoms were relieved. Subretinal fluid pockets were completely resolved in OD, while the ones in OS persisted with some improvement (Fig. 4A–D). The topical dexamethasone dose was reduced, and topical cyclosporine-A 0.05% was started.
Figure 3. Anterior segment photographs recorded in June 2023 showing the fluorescein-stained peripheral ulcerative keratitis in the right eye (A, white arrow) and left extensive epithelial fluorescein staining (B).
Figure 4. Infrared images taken one month after the cessation of erdafitinib (July 2023) revealed the complete resolution of the subretinal fluid pockets in the right eye (A) and the persistence of the ones in the left eye with some improvement (B, white arrows). No remarkable findings were present at the right transfoveal spectral-domain optical coherence tomographic (OCT) section (C), while the thickening of the ellipsoid and interdigitation zones together with subretinal fluid still existed in the left transfoveal spectral-domain OCT (D, red arrow). One month after the resumption of erdafitinib (September 2023); subretinal fluid pockets recurred in both eyes on infrared images (E and F, white arrows) together with the recurrence of the thickening of ellipsoid and interdigitation zones and accompanying subretinal fluid (G and H, red arrows).
Then, the erdafitinib treatment was resumed. At the last visit in September 2023, infrared images and SD-OCT sections demonstrated the recurrence of bilateral multifocal neurosensory detachments and thickening of the ellipsoid and interdigitation zones (Fig. 4E–H). Dry eye was under control and PUK was unrecognisable with the ongoing topical low-dose dexamethasone, cyclosporine-A and artificial tears.
DISCUSSION
The administration of tyrosine kinase inhibitors is not complication-free, despite their selective effects. FGFR signalling plays a crucial role in various states of the eye, including the development, survival, stress response and repair of retinal pigment epithelium (RPE) cells. It also contributes to maturation and survival of the ganglion cells, as well as the regulation of Müller cells in the retina[6]. FGFR inhibition can cause apoptotic protein generation and result in damage or degeneration of the RPE cells, as FGFR is expressed throughout the retina. Consequently, fluid accumulation between the neurosensory retina and RPE may occur and present with an appearance resembling central serous chorioretinopathy (CSC)[1]. Previous reports on erdafitinib-induced ocular complications were listed in Table 1[1,3-15].
During the phase II clinical studies of erdafitinib, Prensky et al.[10] were the first to identify reversible macular lesions on OCT in two patients who presented with blurred vision. One experienced subfoveal retinal detachments with hyper-reflective subretinal material, that resolved one month after discontinuing the erdafitinib. The second patient developed bilateral, multiple foci of retinal detachments with thickening of the photoreceptor layer, showing improvement in the two-week follow-up after cessation of erdafitinib. Both patients experienced ocular issues after the dose escalation.
The clinical presentation of erdafitinib-induced retinopathy has been previously identified as CSC or pseudo-CSC due to its clinical appearance on OCT sections[3,16]. Despite the presence of subretinal fluid and neurosensory retinal detachment; comprehensive examinations using fluorescein angiography (FA) and indocyanine green angiography (ICGA) have revealed no leakage or RPE alterations[5,7,9]. These findings suggest that erdafitinib-related retinopathy may exhibit unique characteristics. Hence, this clinical appearance cannot be called CSC; instead, the term pseudo-CSC may be more suitable. Since the findings were consistent with erdafitinib-induced retinopathy in the present case, we refrained from obtaining FA or ICGA to mitigate the risks associated with dye angiography, due to the patient’s history of nephrectomy.
Fasolino et al.[7] were the first to employ OCT-A in a patient with erdafitinib-induced retinopathy, conducting 6×6 mm scans at the baseline, with one-month and two-month follow-ups after administering erdafitinib. The authors did not report any observable changes in the deep capillary plexus, nor were there significant alterations in choriocapillaris flow void. We performed a 3×3 mm OCT-A in our case but did not observe any remarkable findings.
Francis et al.[17] examined 146 patients with solid tumours who had undergone treatment with various FGFR inhibitors and reported that FGFR inhibitor associated retinopathy was observed in 20 patients (13.7%). Five (25%) were specifically receiving erdafitinib treatment. Six patients (30%) experienced visual complaints concurrent with the subretinal fluid accumulation. Our case did not have any visual symptoms related to the posterior segment involvement.
Due to its high prevalence of erdafitinib-induced retinopathy, an ophthalmic screening protocol has been recommended[3]: A baseline ophthalmological examination including visual acuity, Amsler grid testing, fundoscopy and OCT, before initiating the erdafitinib therapy; Amsler grid monitoring; monthly examinations for the first four months of treatment and every three months afterwards; for an abnormal Amsler grid or any visual abnormalities, examination and dose modifications. Therefore, conducting ophthalmological examinations for patients who will undergo erdafitinib therapy before and during the treatment will facilitate the early detection of potential complications.
The similarity between FGFR inhibitor associated retinopathy and mitogen-activated protein kinase inhibitor (MEK inhibitor) associated retinopathy was underscored. Both share comparable findings and certain ocular problems, notably bilateral, serous elevations typically involving the fovea, and fluid accumulation between the RPE and the interdigitation zone[5]. Although these are typically temporary and usually do not require therapy discontinuation, it is essential to consider the possibility of severe sight-threatening ocular symptomatology in patients with carcinomas being related to a paraneoplastic syndrome[16]. Therefore, it is crucial to distinguish the drug-induced retinopathy from the paraneoplastic syndromes to avoid wrong therapeutic strategies.
Erdafitinib may cause anterior segment complications as well as the posterior segment findings. Bauters et al.[4] reported three cases with anterior segment changes during erdafitinib treatment. One had superficial punctate keratitis and thinning of the corneal stroma. The second case experienced bilateral corneal thinning along with a unilateral corneal ulcer, accompanied by a sudden onset white cataract and angle-closure glaucoma. The third case displayed significant dry eye symptoms and findings. The authors emphasised the importance of high level of awareness regarding the erdafitinib-related anterior segment complications as neglecting these changes could lead to vision loss. The present case developed bilateral superficial punctate keratitis and a PUK in the right eye; both were improved after the cessation of erdafitinib together with the administration of topical treatment. To the best of our knowledge, our case marks the first reported case of PUK in association with erdafitinib therapy.
In conclusion, erdafitinib has the potential to cause various ocular complications. Ocular involvement could affect both anterior and posterior segments concurrently as happened in our case. A high level of suspicion and close monitoring with a collaborative effort seems to be a requisite for every patient receiving erdafitinib therapy.