ABSTRACT
Introduction: Ventricular septal defect (VSD) is a severe complication following acute myocardial infarction (MI) resulting from mechanical disruption of the interventricular septum due to extensive myocardial necrosis. Despite advances in management, the mortality rate approaches 50%. We report a case of a 58-year-old male with VSD following MI who was successfully treated with a delayed surgical approach after haemodynamic support using Impella.
Case description: A 58-year-old man with type 2 diabetes mellitus and hypertension presented with three days of chest pain. Testing revealed late presenting acute anterior ischaemic infarction and left-to-right shunt in the apical ventricular septum. Urgent cardiac catheterisation showed near-total occlusion of the left anterior descending artery. An Impella CP® was placed before angioplasty with a drug-eluting stent to optimise haemodynamics. After a multidisciplinary discussion, the Impella CP® was upgraded to Impella 5.5®, and surgery was delayed allowing for scar formation. The patient remained in the intensive care unit, where he underwent physical therapy, showing improvements in exercise tolerance by the time of surgery. He underwent a left ventriculotomy with a successful repair via an endocardial patch 28 days after initial presentation. Post-operative recovery was uneventful, with the patient discharged five days later, reporting no physical limitations one month post-discharge.
Conclusion: The successful management of VSD post-MI relies on interdisciplinary collaboration, careful timing of surgical intervention and the strategic use of mechanical support devices such as the Impella. This case highlights the potential for favourable outcomes when tailored treatment approaches are employed.
KEYWORDS
Ventricular septal defect, myocardial infarction, mechanical circulatory support, LVAD
LEARNING POINTS
- Given the rarity of ventricular septal defects (VSD) post-myocardial infarction (MI), maintaining a high index of suspicion, particularly in patients with anterior infarcts and other high-risk features, is imperative for ensuring early recognition and management of this life-threatening complication.
- Surgical repair is the treatment of choice for VSD post-MI, offering improved survival rates, particularly when intervention is delayed to allow for myocardial scarring.
- Mechanical circulatory support devices, such as the Impella, can play a crucial role in bridging patients to surgical repair by providing temporary haemodynamic stabilisation. However, timing is vital, and early initiation of mechanical support can prevent the progression of cardiogenic shock and multi-organ failure.
INTRODUCTION
Ventricular septal defect (VSD) is a rare yet grave mechanical complication that can occur following acute myocardial infarction (MI). The development of VSD after MI stems from the mechanical disruption of the interventricular septum due to extensive myocardial necrosis, typically occurring within the first week following the infarction[1]. This rupture establishes a communication between the left and right ventricles, precipitating haemodynamic instability, congestive heart failure and cardiogenic shock. Although the incidence of VSDs post-MI has demonstrated a marked decline from 1–3% in the pre-reperfusion era to approximately 0.2% in the reperfusion era, mortality rates persist at concerning levels, approaching 50%[2].
Surgical closure stands as the treatment of choice, as conservative management is associated with heightened mortality rates. However, the timing of surgical repair necessitates careful consideration, given the peri-infarct myocardial changes, which may not be amenable to successful suturing[1]. While awaiting surgery, medical management focuses on afterload reduction to enhance effective left ventricular stroke volume. We present a case of anterior wall MI complicated by an apical VSD that was successfully treated with surgical repair after 28 days of Impella mechanical support.
CASE DESCRIPTION
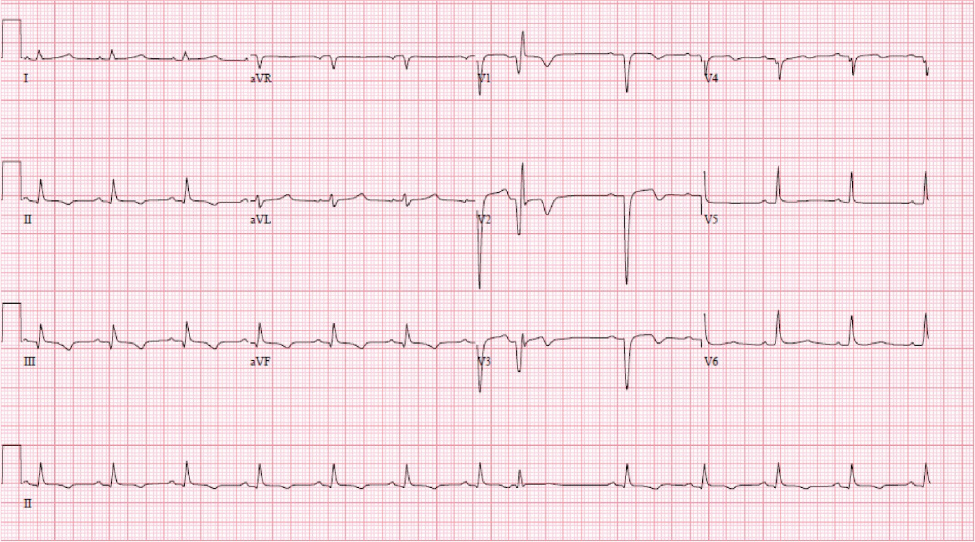
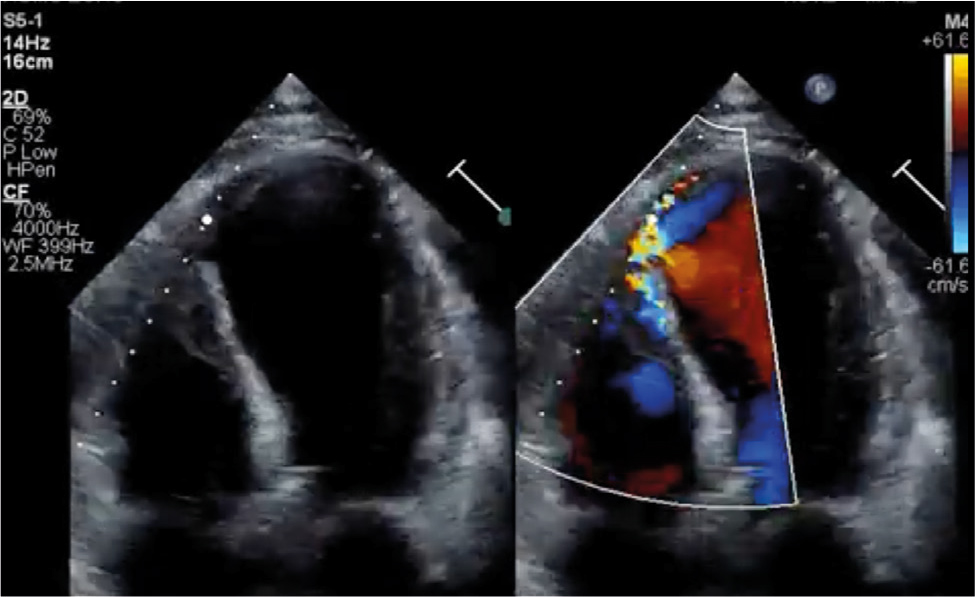
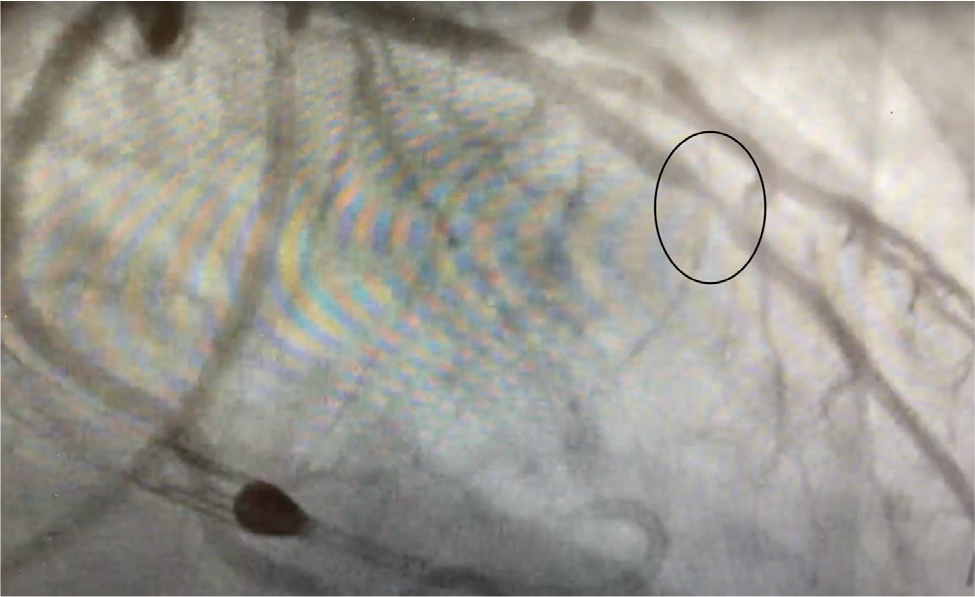
A 58-year-old male with a past medical history of type 2 diabetes mellitus, hypertension and hyperlipidaemia presented to an outside facility, with a 3-day history of stuttering chest pain at home. An electrocardiogram (EKG) on arrival was notable for sinus rhythm and anterior infarct pattern associated with ST elevations, T wave inversions and poor R wave progression across the precordial leads (Fig. 1). Troponins were only mildly elevated at 0.86. A transthoracic echocardiogram (TTE) demonstrated reduced left ventricular ejection fraction (LVEF), apical wall motion abnormalities and a left-to-right shunt across the apical ventricular septum (Fig. 2). Subsequent cardiac catheterisation revealed a 99% stenosis in the mid-left anterior descending artery (LAD) (Fig. 3). A decision was made to place an Impella CP® before percutaneous coronary intervention (PCI) of the LAD lesion in the hope of reducing the shunt across the VSD, and improving biventricular haemodynamics. He tolerated the procedure well with PCI of the LAD lesion, resulting in TIMI 3 flow. Given his VSD, he was transferred to a higher level of care facility for consideration of surgical versus transcatheter repair.
On arrival at our facility, a multidisciplinary team met to discuss the method and timing of the repair. Although surgical repair is a class I indication in a patient, there was careful consideration of timing as the peri-infarcted myocardium required adequate time to scar. Scarring was necessary to allow sufficient time for sutures to hold when patching the VSD was attempted. Additionally, there was apprehension regarding the transcatheter approach given an apically located VSD.
A decision was made to upgrade to an axillary Impella 5.5® and delay surgical intervention. The patient remained in the cardiothoracic intensive care unit (CTICU) working daily with physical therapy, and remarkably, he was able to complete 20 laps of assisted walking around the CTICU up until his surgery.
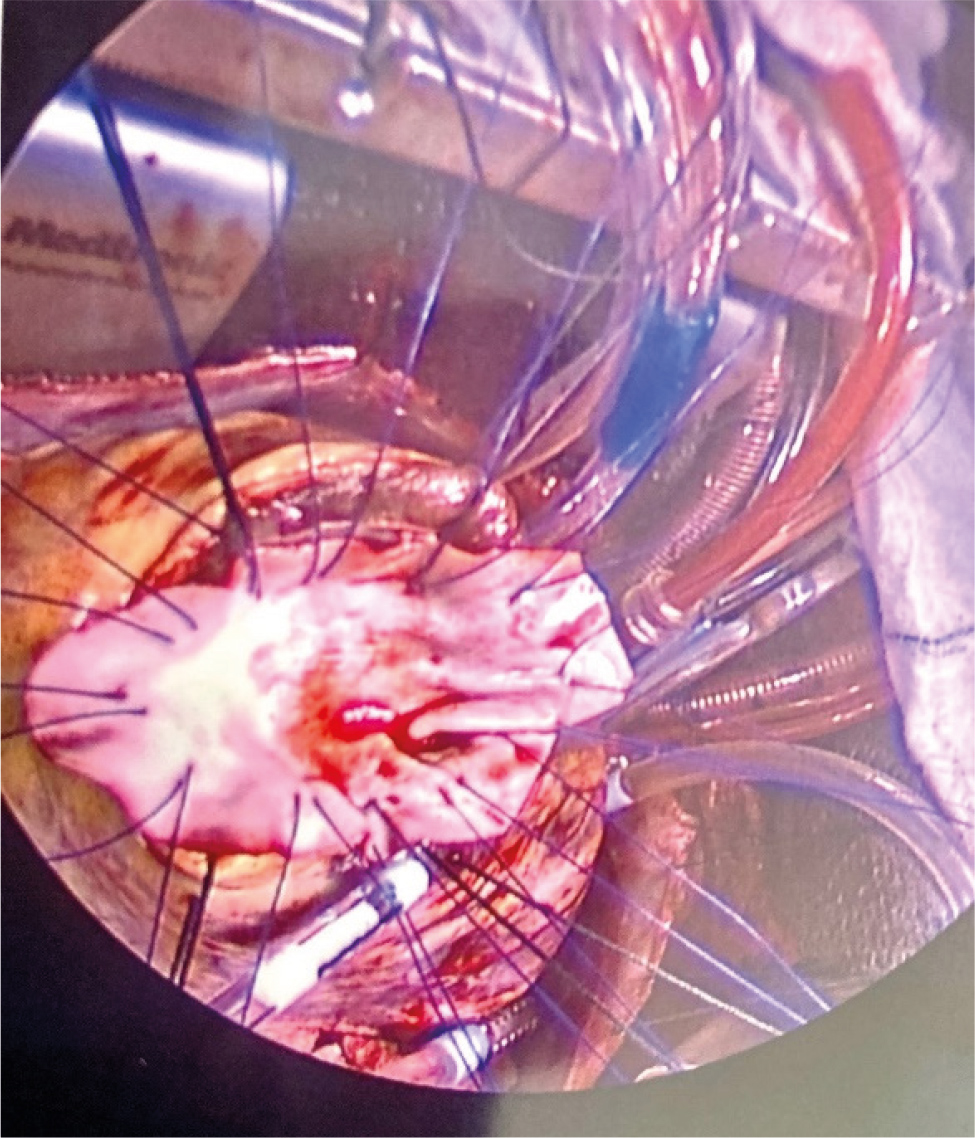
Twenty-eight days after the initial presentation, the patient underwent successful surgical VSD repair with an endocardial patch (Fig. 4). The axillary Impella remained in place for two more days after surgical repair for left ventricular decompression to decrease the risk of ventricular rupture. The patient was discharged five days later, ambulating without any assistance. His post-op TTE demonstrated a well-seated patch without any residual evidence of a leak and a mild improvement of his LVEF. He was seen for a post-op appointment one month later, reporting no limitations with physical activity.
Figure 4. Surgical ventricular septal defect (VSD) repair with an endocardial patch.
DISCUSSION
VSDs are critically important to identify as they can rapidly lead to haemodynamic instability and cardiogenic shock[1]. The literature describes risk factors including old age, female gender, a first MI and an anterior infarct[2,3]. The mechanisms behind the increased incidence in these patients are multifactorial with variable presentations, anatomical differences and hormonal factors likely involved in the interplay.
Conservative management of these patients is associated with extremely high mortality (>90%). Thus, surgical repair remains the treatment of choice, with a mortality rate of around 50%[2]. However, mortality rates post-surgical repair are also variable, ranging from 20% to 100%, depending on the experience and skill of the surgeon[2,4]. Inferior infarction, right ventricular dysfunction and the development of cardiogenic shock are implicated in poor prognoses.
Surgical repair of anterior VSD usually involves making an incision in the left ventricular apex, fitting a pericardial patch, and suturing it to non-infarcted areas. The timing of surgery is a topic of contention, with the American College of Cardiology and the American Heart Association guidelines recommending early surgical intervention; in contrast, several studies have demonstrated improved outcomes with delayed surgery[5]. However, some reports of improved outcomes with delayed surgery may have an inherent bias as patients with unfavourable presentations may be excluded due to early mortality.
Patients with VSD often present with haemodynamic instability, including cardiogenic shock. Mechanical circulatory support (MCS) and medical management in patients awaiting surgical repair can stabilise haemodynamics. In our case, a delayed surgical approach with Impella support provided optimal haemodynamic support while allowing adequate time for scar formation and subsequent successful endocardial repair. However, the timing of Impella initiation is vital to prevent the progression of cardiogenic shock. Previous studies have reported mixed results regarding the use of Impella. A multicentre study involving 28 patients reported high mortality with the use of MCS devices, including Impella. However, the study included severe cases with multi-organ failure[6].
CONCLUSION
Timing is crucial for optimising patient outcomes. Our study demonstrates that early use of Impella for MCS can improve patient outcomes by preventing the progression of cardiogenic shock. Furthermore, a tailored physical therapy programme can help improve surgery outcomes in suitable patients by improving haemodynamic stability and preventing physical deconditioning due to prolonged bed rest. Thus, clinicians can bridge patients to surgical repair by implementing a modified approach of effective circulatory support, medical management, and physical therapy, while minimising the risk of further haemodynamic compromise.