ABSTRACT
The incidence of post-infectious autoimmune diseases has been on the rise following the COVID-19 pandemic. Recently, an autistic patient was admitted to the hospital presenting with a mild upper respiratory system COVID-19 infection. Months after recovery and polymerase chain reaction negativity, the patient developed HEp-2 cell positivity and presented with relapsing polychondritis (RP), a rare autoimmune disease. The mechanism of this autoimmune invasion is ultimately caused by activating a myriad of immune reactions. Lymphocytopenia almost always accompanies various clinical forms of COVID-19; however, it may drive the lymphocytopenia-induced proliferation of autoreactive T cells via the activation of interleukin-6 (IL-6). Moreover, high levels of neutrophils during infection promote autoimmune disease by releasing cytokine and chemokine cascades that accompany inflammation, and neutrophil extracellular traps regulating immune responses through cell–cell interactions. Furthermore, autism spectrum disorder patients display an altered immune system that includes an augmented inflammatory cytokine milieu leading to an increased pro-inflammatory Th1/Th2 ratio. In addition, the pathophysiology of RP is majorly associated with a cell-mediated immune reaction; thus, the predisposing exaggerated immune system of such patients must also be considered as a predisposing factor to the development of post-infectious autoimmune diseases.
KEYWORDS
COVID-19, SARS-CoV-2, relapsing polychondritis, HEp-2 cells, autism
LEARNING POINTS
- COVID-19 infection is a potential trigger for relapsing polychondritis, an autoimmune disease affecting cartilage, and must be considered as a rare post-COVID complication.
- The hyperactive immune system in autism spectrum disorder (ASD) is an important predisposing factor to the induction of more autoimmune diseases after the occurrence of post-infectious dysregulation.
- Lymphocytopenia-induced proliferation possibly initiates the post-infection immune dysregulation.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is an infectious disease associated with coronavirus 2 (SARS-CoV-2) that primarily manifests as a respiratory illness, with symptoms ranging from mild flu-like symptoms to severe pneumonia and acute respiratory distress syndrome (ARDS). Emerging evidence suggests that COVID-19 can lead to the alteration of the immune system with the development of an autoimmune phenomena. The consequence of this immune dysregulation ranges from the production of autoantibodies to the onset of rheumatic autoimmune disease. As described in this case study of a patient who was diagnosed with relapsing polychondritis (RP) shortly after recovering from COVID-19, RP is an immune-mediated systemic inflammatory disease that primarily affects cartilaginous structures of the ears, nose and tracheobronchial tree. The exact pathophysiology of RP remains unclear; however, RP could be considered a Th1-mediated disease as serum levels of interferon gamma (IFN-γ), interleukin 12 (IL-12) and IL-2 parallel changes in disease activity, while the levels of Th2 cytokines do not[1].
CASE DESCRIPTION
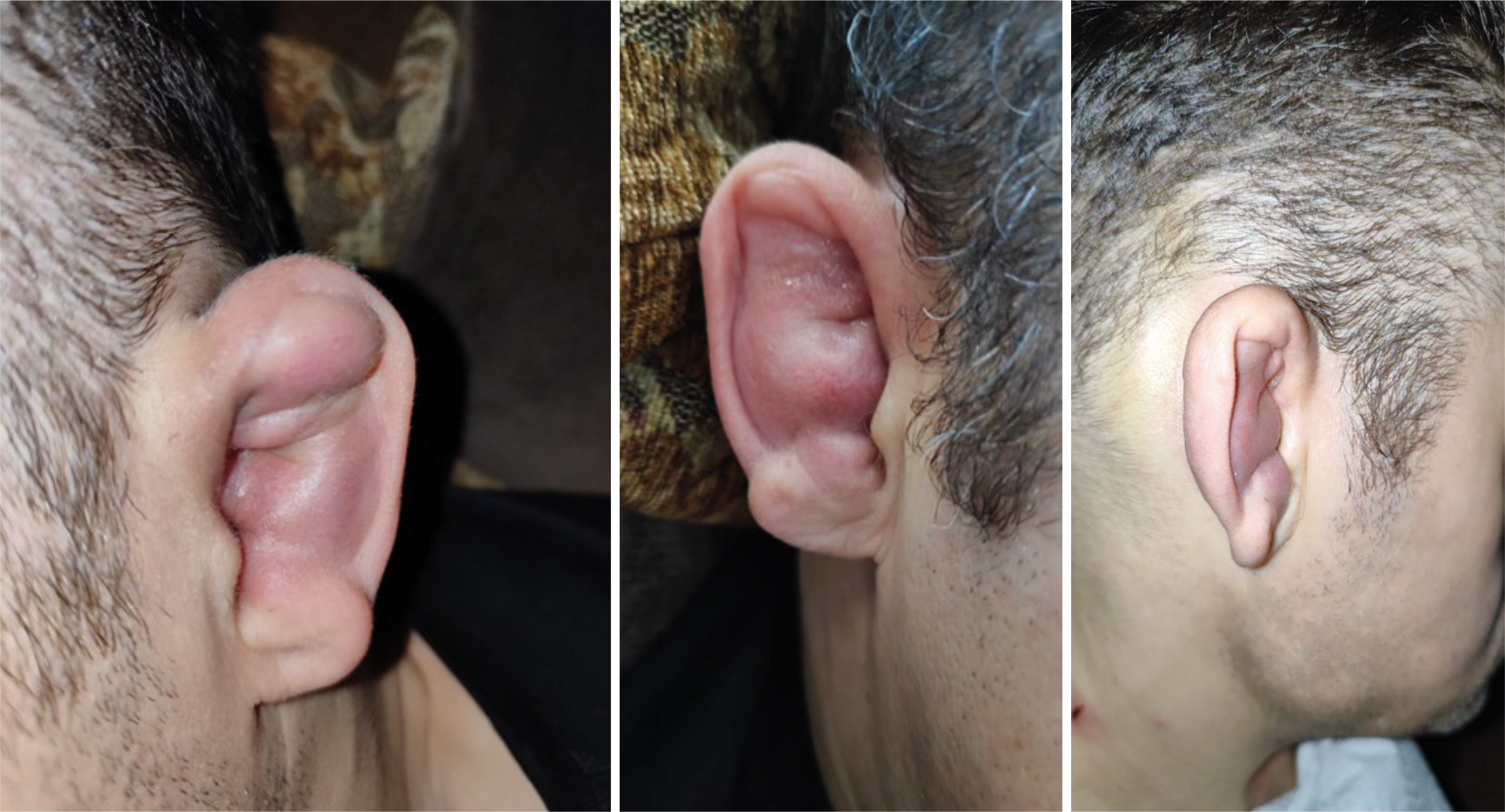
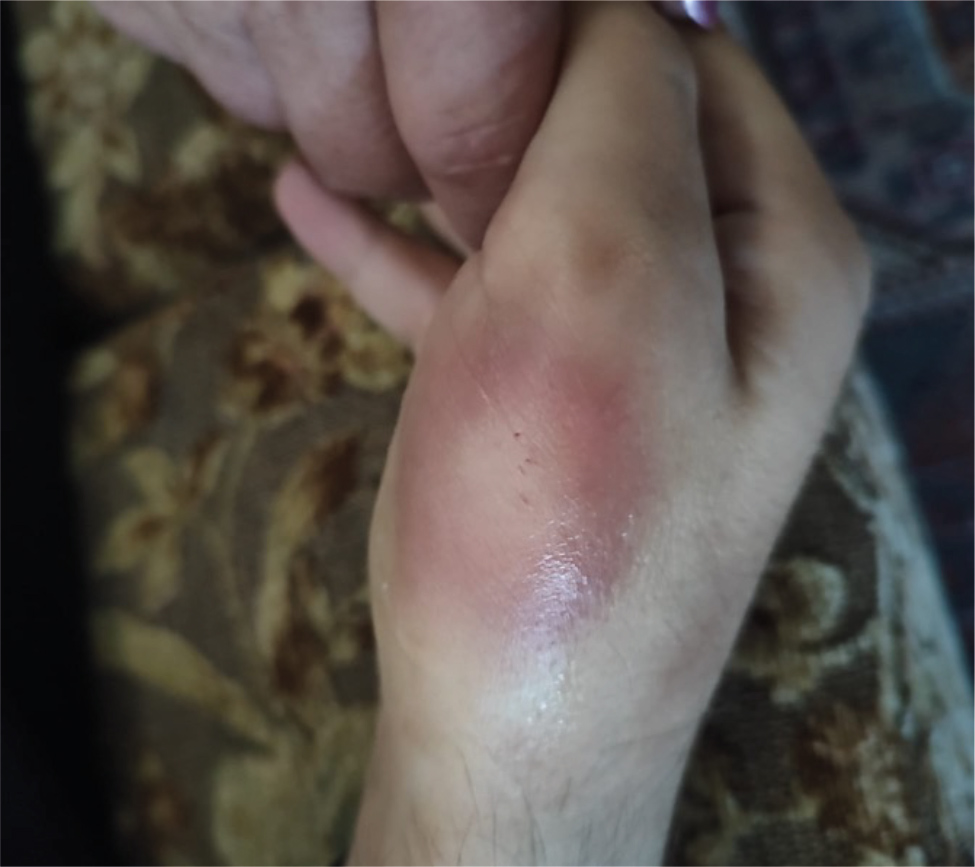
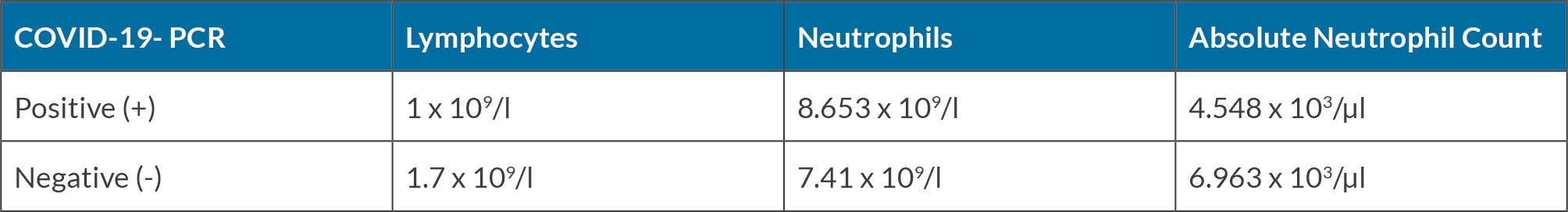
A 42-year-old autistic male patient, with shortness of breath and spot ecchymoses on the body, including the arms and soles of the feet, was admitted to the hospital with a preliminary diagnosis of pneumonia and pulmonary abscess. The patient required hospitalisation and was administered antibiotic treatment for a period of four weeks, which improved his respiratory symptoms and alleviated the bacterial infection’s manifestations. However, before the planned date of discharge, he tested positive for SARS-CoV-2, requiring an extension of his hospitalisation period. Our patient had a history of mild anaemia with slightly low haemoglobin levels of 11.8 g/dl, thrombocytopenia, vitamin D deficiency and other unspecified vitamin deficiencies. Moreover, he also had previous diagnoses of peripheral vascular diseases, recurrent oral sores, dermatitis, constipation, myalgia, and a previous history of a severe COVID-19 infection which required 8 weeks of hospitalisation 2 years previously. Following three weeks of full recovery and polymerase chain reaction (PCR) negativity, the patient returned for consultation regarding right side auricular chondritis presenting with complaints of redness, swelling and pain (Fig. 1). The initial attack spontaneously resolved; however, it was followed by a recurrence of symptoms in the contralateral ear 20 days later, which required steroid treatment to relieve the patient’s discomfort. Two months after the first episode, the patient experienced subsequent attacks in the first carpometacarpal joint and then the temporomandibular joint of the face (Fig. 2). With further testing, fluorescent antinuclear antibodies in HEp-2 cells were detected showing a granular pattern with titre (1/80). The patient’s lymphocyte and neutrophil count is recorded in Table 1 during periods of PCR positivity and negativity.
DISCUSSION
The misregulated restoration of the immune system post-infection is an important trigger in the induction of an autoimmune disease. A competent immune system is required to maintain immune haemostasis and prevent this development. An expected immune response to all clinical forms of COVID-19 includes lymphocytopenia. Under the lymphopenic condition, IL-6 (a consequence of antigen-presenting cells activation via infection) may drive the lymphocytopenia-induced proliferation of autoreactive T cells. The lymphopenia-induced proliferation of autoreactive T cells and the production of autoantibodies increase autoimmunity that may further develop into autoimmune diseases[2]. In this patient, the lymphocyte count increased from 1 × 109/l (during early infection) to 1.7 × 109/l (Table 1) after recovery from COVID-19 infection and during the initial presentation of RP.
Furthermore, the activation of toll-like receptors and the complement system perpetuates NETosis and leads to autoantibody formation, predisposing individuals to systemic autoimmunity, including reactive arthritis and connective tissue disorders[3]. Within this spectrum of autoimmune effects, RP emerges. This association existed in this case when presented in our clinic, where further assessment revealed fluorescent antinuclear antibodies in HEp-2 cells, using a method for screening systemic autoimmune rheumatic diseases, including rheumatoid arthritis and RP[4]. Thus, we acknowledge that RP might be a sequel in post-COVID-19 patients after the stimulation of specific autoantibodies as a consequence of the number of damaged cells by SARS-CoV-2 virus.
Moreover, it is essential to consider that the patient we are presenting is a patient with ASD, therefore it is critical to consider relevant immune system alterations and that ongoing immune dysregulation persists in such patients. Altered immune regulation in ASD patients includes an increased inflammatory cytokine milieu leading to an increased pro-inflammatory Th1/Th2 ratio[5]. Different studies have shown the pattern of cytokine elevation in RP compared to rheumatoid arthritis. Results showed that levels of three serum cytokines were significantly higher in RP patients than in age- and sex-matched controls. All three cytokines are pro-inflammatory chemokines, characteristically involved in activating the monocyte and macrophage lineage, and neutrophils in the case of interleukin-8. Moreover, a major role in cell-mediated immune response in the in the pathophysiology of RP is known[6]. Therefore, the enhanced pro-inflammatory Th1/Th2 ratio in ASD patients could be a predisposing factor to the formation of RP.
CONCLUSION
A clear association between COVID-19 and the progression of autoimmune diseases such as RP has been established. The formation of autoimmune disease occurs after failing to maintain steady-state haemostasis in immune regulation, commonly triggered by viral infections. The induction of lymphocytopenia-induced proliferation of autoreactive T cells post-viral infection is significant in initiating autoimmune dysregulation. The formation of HEp-2 autoantibodies is directly associated with the mass cellular destruction of SARS-CoV-2 infected cells. Furthermore, in the case of ASD, the consideration of a hyperactive immune system must be recognised.