ABSTRACT
This report presents a 57-year-old female with a history of dyslipidaemia, intolerant to statins and currently managed on evolocumab. Despite a healthy lifestyle, lipid panel abnormalities persisted, leading to an investigation that revealed heterozygous mutations in the ABCG8 gene, confirming a diagnosis of sitosterolaemia. The patient’s unique response to lipid-lowering medications typified this rare disorder, necessitating specialised genetic testing for diagnosis. Management involved dietary modifications and the introduction of ezetimibe, evolocumab and atorvastatin, demonstrating the personalised nature of treatment. The case underscores the importance of considering sitosterolaemia in unexplained lipid abnormalities and highlights the challenges in diagnosis and management. Ongoing research is crucial for refining diagnostic and therapeutic strategies for this clinically significant disorder, emphasising the need for a multidisciplinary approach to patient care.
KEYWORDS
Dyslipidaemia, sitosterolaemia, ezetimibe, evolocumab
LEARNING POINTS
- Recognise the significance of considering sitosterolaemia in differential diagnosis for unexplained lipid abnormalities.
- Understand the challenges in diagnosing and managing sitosterolaemia, especially in patients with atypical responses to conventional lipid-lowering therapies.
CASE DESCRIPTION
The patient is a 57-year-old female with a history of dyslipidaemia and a family history significant for multiple cardiovascular events. She is intolerant to multiple statins due to side effects and lack of adequate response and was currently on evolocumab. She presented in the outpatient setting to the preventative cardiology specialist clinic for further evaluation. She did not have any cardiovascular-related complaints. She reported a healthy lifestyle, adhering to a low-fat, low-carb diet and engaging in regular aerobic exercises. The patient has been prescribed lipid-lowering medications since her 30s. She attempted multiple statins with different doses, but often reported muscle aches. Rosuvastatin was changed to evolocumab due to side effects and the lack of appropriate response, which is being well tolerated. The lipid panel prior to initiation of evolocumab revealed elevated total cholesterol of 307 mg/dl (normal range 125–200 mg/dl), low-density lipoproteins (LDL) of 208 mg/dl (normal range <100 mg/dl), high density lipoprotein (HDL) of 78 mg/dL (normal range >50 mg/dl) and triglycerides of 107 mg/dl (normal range <150 mg/dl). A follow-up lipid panel indicated a suboptimal response, with LDL of 251 mg/dl. Physical examination revealed BP 135/79, pulse 75 and oxygen saturation 97%. The examination was unremarkable overall except for minimal bilateral superior corneal arcus and a grade 1 holosystolic murmur near the cardiac apex. Past medical history included mild to moderate mitral regurgitation and mild tricuspid regurgitation, aortic valve sclerosis with mild aortic insufficiency and a prior episode of deep-vein thrombosis. The thrombotic episode was managed with a 6-month anticoagulation regimen and was suspected to be associated with oral contraceptive use. Family history is significant for multiple cardiovascular events. Several paternal aunts and uncles experienced heart attacks and strokes in their 60s. While her father never exhibited these events, he was on cholesterol-lowering medication.
Differential diagnosis:
- Hypothyroidism, diabetes mellitus and nephrotic syndrome – ruled out with prior appropriate testing;
- Familial hypercholesterolemia and polygenic hypercholesterolemia – however, they tend to have a more favourable response to statin therapy;
- Lipoprotein lipase or hepatic lipase deficiency.
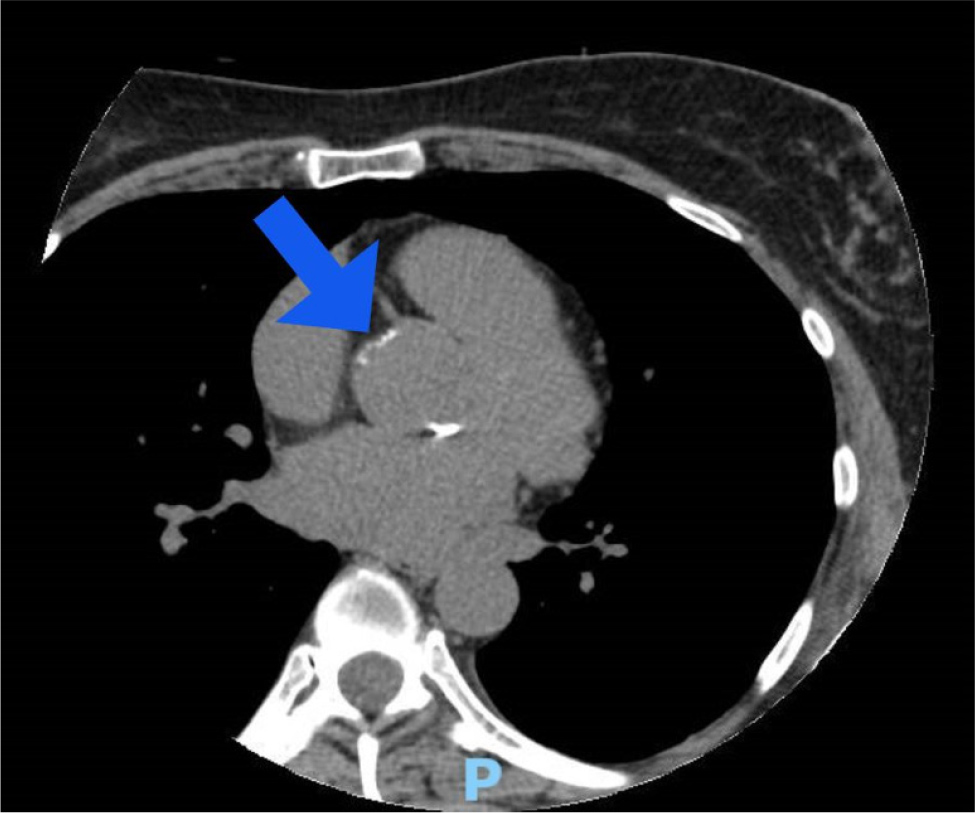
HbA1c, liver and thyroid function tests were normal. A genetic work-up identified two heterozygous mutations in the ABCG8 gene, confirming a diagnosis of sitosterolaemia (phytosterolaemia). A CT calcium score used for risk stratification reveal a total Agatson score of 14, placing the patient at the 80th percentile when adjusted for her age, gender and race/ethnicity (Fig. 1). The evolocumab injection site was adjusted to the thigh and ezetimibe 10 mg daily was introduced.
Figure 1. A CT cardiac calcium scoring showing a small atherosclerotic plaque at the left main coronary artery ostium.
DISCUSSION
This case delves into sitosterolaemia, an autosomal recessive disorder disrupting lipid metabolism with elevated plant sterol levels, notably sitosterol and campesterol, leading to premature coronary artery disease. Sitosterolaemia typically presents with clinical features that can be similar to other lipid disorders. Patients commonly exhibit tendon xanthomas, a characteristic shared with familial hypercholesterolaemia.
Additionally, they may show signs such as corneal arcus and xanthelasma, although these are less frequent. Other non-specific symptoms can include abdominal pain and joint pain. In advanced stages of the disease, splenomegaly and hepatomegaly may also manifest.
The diagnostic challenges stem from its phenotypical heterogeneity and rarity, often eluding conventional lipid panels. Specialised techniques such as gas-liquid chromatography or genetic testing become crucial. The discovery of ABCG8 gene mutations in the presented patient aided the diagnosis, highlighting the need to consider sitosterolaemia in unexplained lipid abnormalities. Detecting elevated plasma sitosterol levels through advanced techniques is pivotal for diagnosis[1-4].
Management of sitosterolaemia involves diverse strategies:
- Dietary modifications: essential for restricting non-cholesterol sterol intake, involving limitations on certain foods while allowing others. Dietary changes may have limited impact on homozygotes[5].
- Medical interventions: ezetimibe is a primary treatment, reducing sterol absorption. Bile acid sequestrants such as cholestyramine may be considered. Statins are ineffective in sitosterolaemia, distinguishing it from familial hypercholesterolaemia[6,7].
- Surgical measures: partial ileal bypass and liver transplants show efficacy, particularly in cases of ABCG8 mutation-related liver cirrhosis, suggesting potential for correcting the biochemical abnormality[8,9].
In our case, the patient’s subpar response to multiple lipid-lowering medications typified sitosterolaemia. Nevertheless, introducing ezetimibe and evolucumab yielded substantial LDL cholesterol reductions, emphasising the personalised nature of sitosterolaemia treatment. The patient continued with a heart-healthy lifestyle with sitosterolaemia dietary modifications, which include low plant sterols.
A repeat lipid panel after 4 weeks of the above modifications revealed improved results with total cholesterol of 196 mg/dl, LDL of 102 mg/dl and HDL of 72 mg/dl. Also, atorvastatin 10 mg daily was added to her regimen, to further optimise her lipid profile to achieve an optimal LDL cholesterol target of less than 70 mg/dl. Ongoing research continues to refine the long-term prognosis for sitosterolaemia patients. Regular monitoring, as demonstrated here, is crucial for therapeutic efficacy and lipid level regulation. Moreover, a multidisciplinary approach integrating lipid specialists, genetic counsellors and dietitians, may be pivotal for these patients’ holistic care and heart-healthy lifestyle promotion.
CONCLUSION
This report underscores the need to consider sitosterolaemia in patients with unexplained lipid abnormalities, especially when conventional therapies yield atypical responses. Genetic testing proves crucial for identifying underlying genetic determinants. Management involves dietary adjustments, personalised medical interventions and occasionally surgery. Early detection and comprehensive care empower patients to mitigate the risk of premature coronary artery disease, enhancing overall quality of life. Ongoing research is vital for refining diagnostic and therapeutic strategies for this rare but clinically significant disorder.