ABSTRACT
Background: Wellens’ syndrome is characterised by a history of chest pain with an abnormal electrocardiogram (EKG), demonstrating biphasic or deeply inverted T waves in leads V2–3 (may extend to involve all precordial and lateral limb leads – the type B Wellens’ pattern). A Wellens’ EKG pattern is considered highly specific for critical stenosis involving the ostial/proximal left anterior descending artery (LAD). However, there are no reported cases of an association of a Wellens’ EKG pattern with myopericarditis. Here, we present such a rare case.
Case description: A thirty-one-year-old female with known essential hypertension and psoriatic arthritis presented with a constant, central chest pain radiating to the shoulders and back. The patient’s physical examination was unremarkable at presentation other than elevated blood pressure at 170/68 mmHg. An EKG at presentation demonstrated deep symmetric T-wave inversions in anterolateral leads with elevated high-sensitivity troponin, and an elevated erythrocyte sedimentation rate. The patient was referred to the cardiac catheterisation laboratory for concerns of a Wellens’ EKG pattern; however, invasive angiography demonstrated only obtuse marginal branch disease – no LAD disease was noted. Cardiac magnetic resonance (CMR) imaging confirmed the diagnosis of myopericarditis and absence of myocardial infarction. The patient was medically managed and discharged home in a stable condition.
Conclusion: In literature and established clinical practice, the Wellens’ EKG pattern is considered highly concerning for critical ostial/proximal LAD stenosis. However, we now propose that myopericarditis may be considered in a differential diagnosis for this EKG pattern.
KEYWORDS
Myopericarditis, Wellens’ syndrome, coronary artery disease
LEARNING POINTS
- Wellens’ syndrome is characterised by a history of chest pain with an abnormal electrocardiogram (EKG), demonstrating biphasic or deeply inverted T waves in leads V2–3.
- A Wellens’ EKG pattern is considered highly specific for critical stenosis involving the ostial/proximal left anterior descending artery (LAD).
- Association of Wellens’ pattern EKG has been described in association with various other pathologies; however, its association with acute myopericarditis has not been well described.
INTRODUCTION
Electrocardiogram (EKG) findings of biphasic T waves or deep T-wave inversions in leads V2 and V3 (type A Wellens’ pattern) in patients with history of angina are termed Wellens’ syndrome[1,2]. Type B Wellens’ EKG pattern may extend to involve all the precordial leads (V1–V6), as well as the lateral leads (I and aVL)[1-3]. The Wellens’ ECG pattern has a known strong association with ostial/proximal left anterior descending (LAD) coronary artery occlusion[1-3]. Patients presenting with Wellens’ syndrome require antiplatelet agents and early coronary revascularisation in addition to other guideline-directed medical therapy and is considered life-threatening[1-3]. However, Wellens’ EKG pattern in other disease states such as myopericarditis has not been described. Here, we present an interesting case of a patient presenting with a Wellens’ syndrome-like clinical picture in the setting of underlying myopericarditis.
CASE DESCRIPTION
A 31-year-old obese female with history of pre-eclampsia (2 years previously) and subsequent hypertension, anxiety, depression, hypothyroidism, and psoriatic arthritis on guselkumab presented to hospital for evaluation of chest pain. The patient described her chest pain as a constant, central chest pain ongoing for two hours, 7 out of 10 in intensity, radiating to the back and shoulders. There were no aggravating or relieving factors. There were no other associated symptoms, and the patient denied any recent viral prodrome. The patient described a similar, brief, and self-resolving episode of chest pain a week prior. The patient denied any family history of premature coronary artery disease or sudden cardiac death. She was a never-smoker and denied any use of alcohol or other recreational drugs. The remainder of her medical history was non-contributory.
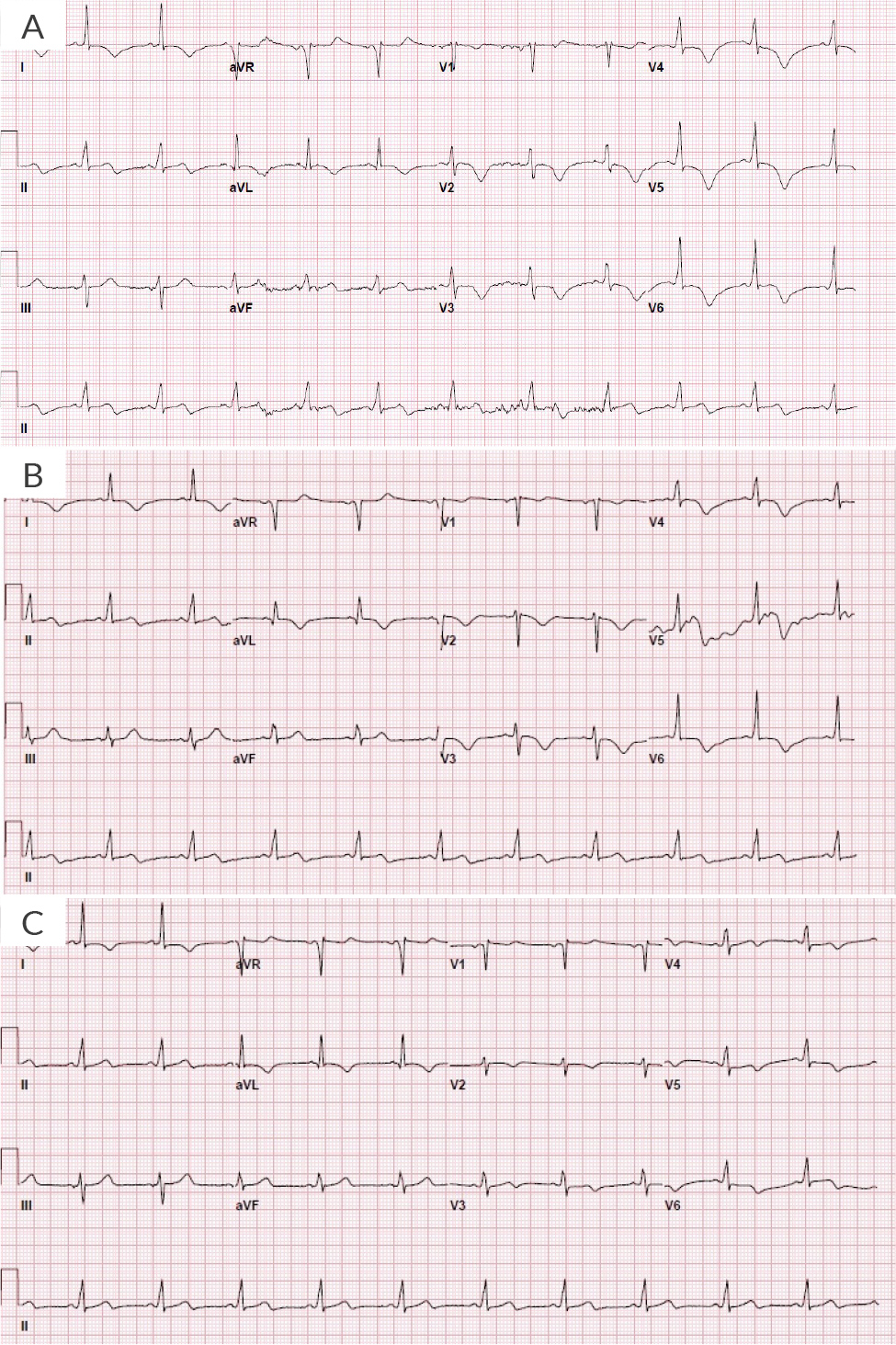
Upon arrival at the emergency department at an outside hospital, she was afebrile with blood pressure of 170/68 mmHg; heart rate was 68 beats per minute, respiratory rate was 18 breaths per minute, and she was saturating 99% on room air. She was not in any acute distress on examination. Lungs were clear to auscultation. Heart sounds were audible for S1 and S2, without any murmurs, gallop rhythms or rubs. There was no jugular venous distention or peripheral oedema, and the remainder of her examination was unremarkable. Initial laboratory work revealed haemoglobin 12.9 g/dl, troponin-I 0.51 ng/ml (normal <0.04 ng/ml), pro-brain natriuretic peptide 1,685 pg/ml, D-dimer 182 ng/ml, erythrocyte sedimentation rate 52 mm/hr, haemoglobin A1C 4.9%. Total cholesterol was 156 mg/dl, triglycerides 204 mg/dl, high-density lipoprotein 31 mg/dl and low-density lipoprotein 84. Her remaining laboratory test results were unremarkable, and chest X-ray was negative for any acute findings. An electrocardiogram (EKG) showed normal sinus rhythm with a ventricular rate of 62 with anterior-lateral deep and symmetric anterior T-wave inversions extending laterally into I/aVL (Figure 1A), and persistent on two-hour repeat EKG (Figure 1B).
Figure 1. Electrocardiogram A) Upon arrival at the hospital; B) Persistent changes two hours after initial presentation; C) Persistent changes at day 2.
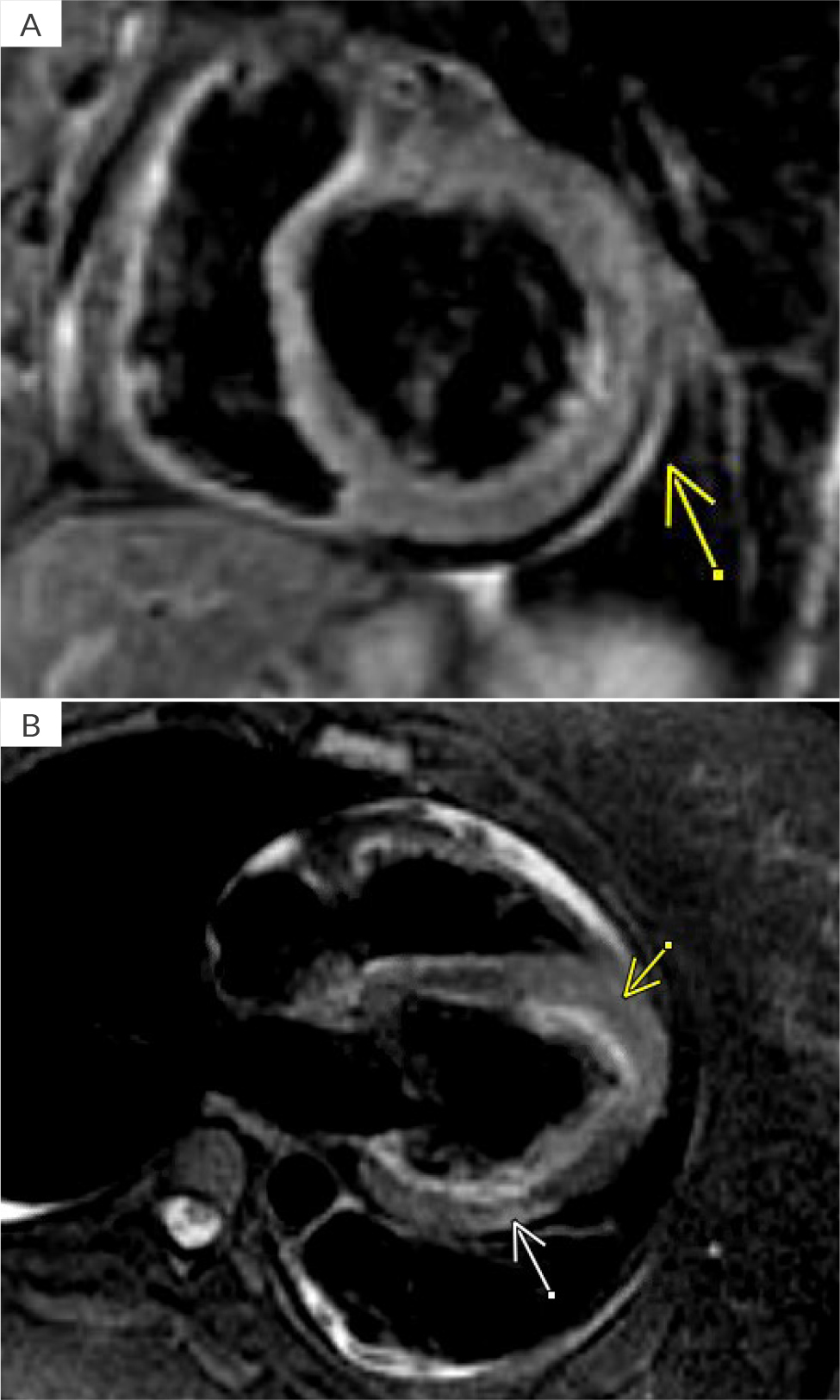
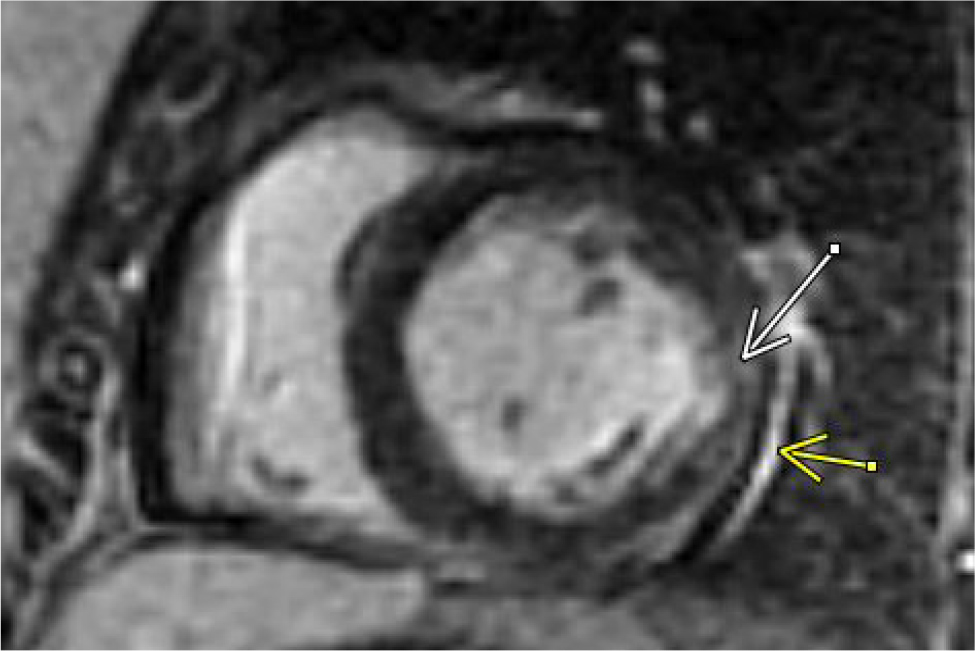
Due to ongoing chest pain, EKG findings and borderline troponin elevation in the setting of risk factors (obesity, hyperlipidaemia, hypertension, and pre-eclampsia), acute myocardial infarction was considered. The patient was given aspirin and started on a heparin drip. Due to elevated D-dimers, a computed tomography angiogram of the chest was performed and was negative for pulmonary embolism or aortic dissection and showed trace pericardial effusion. She was transferred to our facility for left heart catheterisation. High-sensitivity troponin T at our facility was elevated at 527 ng/l – it peaked at 767 ng/l and then trended down (reference range 0–14 ng/l). Invasive coronary angiography was performed and the proximal segment of the inferior division of the obtuse marginal branch demonstrated focal diffuse narrowing. At the time, that was thought to represent a stable medical disease. Other differentials for those findings included focal spasm versus dissection. However, a computerised tomography scan on the subsequent day demonstrated completely normal coronary arteries with a zero-calcium score. There was no evidence of coronary vasospasm, intraluminal thrombus or any other aetiology on invasive angiography that would explain Wellens’ EKG findings (video 1, 2 and 3). A transthoracic echocardiogram (TTE) demonstrated a normal left ventricular (LV) ejection fraction of 55–59% with apical septal hypokinesis, with a depressed global longitudinal strain of -13.2%. Subsequently, the patient underwent inpatient cardiac magnetic resonance imaging (CMR) on day 2, which demonstrated mild global hypokinesis of the LV with focal mid-inferolateral and apical septal segments with moderate hypokinesis. A 3D volumetric left ventricular ejection fraction (LVEF), calculated using short axis stack on steady-state free precession imaging, was 45%. There was a small, circumferential pericardial effusion. On triple inversion T2-weighted oedema sequence, there was significant hyperintensity involving the parietal pericardium along the base to mid-inferolateral surface of the LV. Additionally, there was focal, mostly dense, epicardial hyperintensity involving mid-inferolateral myocardium but much more diffuse mid-anteroseptal, apical septal and apical cap segments concerning for acute myopericardial inflammation/oedema (Figures 2A and B). On gadolinium enhancement sequences, there was both early and delayed (late gadolinium enhancement – LGE) patchy/diffuse myocardial enhancement involving mid-anteroseptal, apical septal, apical cap and mid-inferolateral segments as well as pericardial LGE along the base to mid-inferolateral LV (Figure 3). Importantly, there was complete sparing of the LV subendocardial zone on LGE sequences, which ruled out myocardial infarction as an aetiology. Overall, the findings were consistent with myopericarditis with mild LV dysfunction, and small pericardial effusion without an infarction pattern.
Video 1. Left anterior oblique, caudal view. Note diffuse narrowing of proximal segment of inferior branch of the obtuse marginal.
Video 2. Left anterior oblique, cranial view. Note absence of LAD atherosclerosis, spontaneous dissection or spasm.
Video 3. Non-dominant right coronary artery without any disease.
Figure 2. Triple inversion T2 weighted oedema sequence. A) Pericardial hyperintensity, yellow arrow; B) Subepicardial hyperintensity involving mid-inferolateral segment (white arrow). Note apical septal and apical cap hyperintensity (yellow arrow).
Figure 3. Late gadolinium sequence demonstrating pericardial LGE (yellow arrow) and myocardial LGE (white arrow); note zone of subendocardial sparing.
Due to persistent EKG changes on day 2 (Figure 1C), a coronary computed angiography was requested which ruled out any spontaneous LAD artery dissection; an examination was otherwise consistent with cardiac catheterisation findings. The patient had a zero coronary artery calcium score, and no intraluminal coronary thrombus was seen.
The patient was treated for side branch disease and mild LV systolic dysfunction with aspirin, statins, beta blocker and angiotensin receptor blocker, along with colchicine for myopericarditis. Antinuclear antibody testing was negative. Her pressures normalised and once symptoms were controlled, she was discharged home in a stable condition with advice for outpatient cardiology and rheumatology (to work-up for autoimmune causes) follow-up.
DISCUSSION
Wellens’ syndrome, also known as the “widow-maker”, has been a well-known entity since 1982 when it was first identified as predictor of LAD artery occlusion[1,2] by the late Hein Wellens (2020). It is categorised into type A (with biphasic T waves in leads V2 and V3) and type B (with deep T-wave inversions in leads V2 and V3 that may extend to involve all precordial and lateral limb leads). Wellens’ syndrome is diagnosed in a patient with a history of angina pectoris with electrocardiographic (EKG) findings of biphasic T waves or deep T-wave inversions in leads V2 and V3. This is along with isoelectric or minimal (< 1 mm) ST elevations, normal R wave progression and in the absence of precordial Q waves, with normal or slightly elevated cardiac biomarkers[3].
Since the initial findings, there have been multiple reports suggesting an association of Wellens’ EKG pattern findings with the use of recreational drugs (or with immunotherapy agents, pulmonary embolism, myocardial bridge, acute pancreatitis, acute cholecystitis, sepsis-related takotsubo cardiomyopathy and even porphyria[4]). This gave rise to the term pseudo-Wellens’ syndrome (emphasising no critical LAD stenosis despite the presence of pertinent EKG findings). However, association of myo-pericarditis with Wellens’ EKG changes has not been described in the literature.
Myopericarditis refers to inflammatory disease involving the pericardium, which extends to the myocardial layer of heart leading to elevation in cardiac biomarkers[5]. Viral infection is the most common cause including coxsackieviruses, adenoviruses, cytomegaloviruses, human herpesvirus 6 and parvovirus B-19[5]. More recently, SARS-CoV-2 infection (COVID-19)[6] as well as the vaccination[7] have been identified as aetiologies for myopericarditis[8].
Clinical presentation of myopericarditis is variable, depending on the extent of myocardial involvement. In symptomatic patients fever, myalgia and gastrointestinal symptoms predominate initially. Pleuritic chest pain, decreased exercise capacity and palpitations are commonly reported[5]. Cardiac arrhythmias in myopericarditis are common (> 60%)[5].
Diagnosis of acute myopericarditis remains a challenge due to its non-specific and variable presentation. Classic progressive EKG changes of acute pericarditis include diffuse ST elevation (concave upwards), and PR segment depression. Subsequently, ST segments and PR depressions tend to normalise and diffuse T-wave inversions develop, which may persist. There are no specific myocarditis-related EKG changes. Myopericarditis may present with atypical EKG changes including localised ST elevation or T-wave inversions before normalisation of the ST segment. However, Wellens’ EKG changes in patients with acute myopericarditis as presented in our case[5,9] have not been described.
Imaging modalities such as echocardiogram often provide adjunctive information such as presence of pericardial effusion, impaired LV myocardial function, presence, or absence of regional wall motion abnormalities; tissue Doppler may demonstrate evidence of myocardial involvement. In our case, it showed apical septal hypokinesis with an LVEF of 50–55%. CMR is superior in tissue characterisation, and can reveal a thickened and/or inflamed pericardium, pericardial effusion and myocardial inflammation, and can rule out subendocardial or transmural infarction[5] as seen in our case. Endomyocardial biopsy is the gold standard for the diagnosis of myocarditis; however, it carries significant risk of perforation and embolisation, and is rarely performed. Also, it may not be diagnostic in patchy myocardial involvement. Hence, its use is limited to cases of diffuse/significant myocardial involvement with LV dysfunction, or a malignant course of LV systolic dysfunction or when symptoms are not responsive to conventional medical management[5].
Hussain et al. described a case of myocarditis in association with the Wellens’ EKG pattern in a 19-year-old patient without any coronary risk factors[10]. On the contrary, our patient had hypertension, hyperlipidaemia, obesity (multiple risk factors for coronary artery disease) and was found to have combination of myopericarditis instead of just myocarditis. This again emphasises that myopericarditis may be considered in differential diagnosis of Wellens’ syndrome/Wellens’ EKG pattern.
Myopericarditis without significant LV failure is treated similarly to acute pericarditis, with high-dose, non-steroidal anti-inflammatory drugs and colchicine[5,9]. Steroids are considered only after failure with other therapies as steroid therapy is independently associated with recurrence[5,9]. Intravenous immunoglobulins have also been used; however, without sufficient supporting data. In addition to this, based on evidence, activity and alcohol restriction are recommended along with regular close follow-up visits to reduce the risk of arrhythmia and progressive LV dysfunction, respectively. Myopericarditis may have varied prognosis depending on underlying aetiology[5,9].
CONCLUSION
Our patient had evidence of diffuse narrowing involving the proximal segment of an inferior branch of obtuse marginal artery on an invasive angiography and coronary computerised tomography scan. Classically, side branch disease is unlikely to explain the Wellens’ EKG pattern and the absence of an acute myocardial infarction pattern confirmed via high-resolution, multiparametric CMR makes an intracoronary explanation unlikely. The findings in our case suggest that there is more to the Wellens’ syndrome than is already known and we propose adding myopericarditis to the differential diagnosis of Wellens’ EKG pattern.