ABSTRACT
Background: Atypical myxoma has been reported in various locations in the heart, however, myxoma involving the pulmonary valve is rare. Here we present a case of pulmonic valve myxoma which was resected via a percutaneous approach.
Case Report: A 66-year-old female with known metastatic adenocarcinoma of the lung, and chronic obstructive pulmonary disease presented with acute onset shortness of breath for two days. The patient experienced respiratory arrest en-route to the hospital and required intubation. Computed tomography angiography (CTA) of the chest revealed a new 1.4 x 1.6 cm intracardiac mass along the pulmonary valve. Further evaluation with cardiac magnetic resonance imaging revealed it to be a large vascular tumor on the ventricular side of the pulmonary valve, attached with a narrow stalk. Due to high surgical risk, the patient underwent transesophageal echocardiographic guided percutaneous removal of the mass. Pathology confirmed the mass to be a myxoma.
Conclusion: Atypical myxoma should be considered in the differential diagnosis of valvular masses. Percutaneous resection of valvular masses may be feasible in high-risk surgical patients.
KEYWORDS
Atypical myxoma, pulmonary valve, percutaneous removal, malignant and benign cardiac tumors, case report
LEARNING POINTS
- Pulmonary valve myxoma is a rare condition and the literature on the characteristics and treatment options for pulmonary valve myxoma is limited.
- Our patient was treated with a minimally invasive treatment approach: removal of a tumor with intra operative transesophageal echocardiographic guidance using AngioVac and Flow Triever catheters.
- Percutaneous resection of valvular masses may be feasible in high surgical risk patients.
INTRODUCTION
Myxoma is the most common benign primary tumor of the heart[1-4] with the potential to embolize and cause life-threatening complications[2-4]. Though myxoma typically involves the interatrial septum, involvement of the mitral valve, aortic valve, and superior vena cava have been reported[1-4]. However, cardiac myxoma arising from the pulmonary valve is rare. We present a case of pulmonary valve myxoma which was treated via percutaneous approach.
CASE PRESENTATION
A 66-year-old female with known metastatic adenocarcinoma of the lung, on immunotherapy, was brought to hospital intubated due to progressive dyspnea and acute respiratory failure. Her history was significant for chronic obstructive pulmonary disease (COPD) with a 30 pack-year smoking history, and chronic hypoxic respiratory failure and she was on 4 liters of supplemental oxygen.
On presentation, her vital signs were as follows: blood pressure 130/84 mmHg, heart rate 84 beats per minute, temperature 36.5°C, and respiratory rate 18 breaths per minute. On examination, the patient was intubated, sedated and had bilateral wheezing. The rest of the examination was unremarkable.
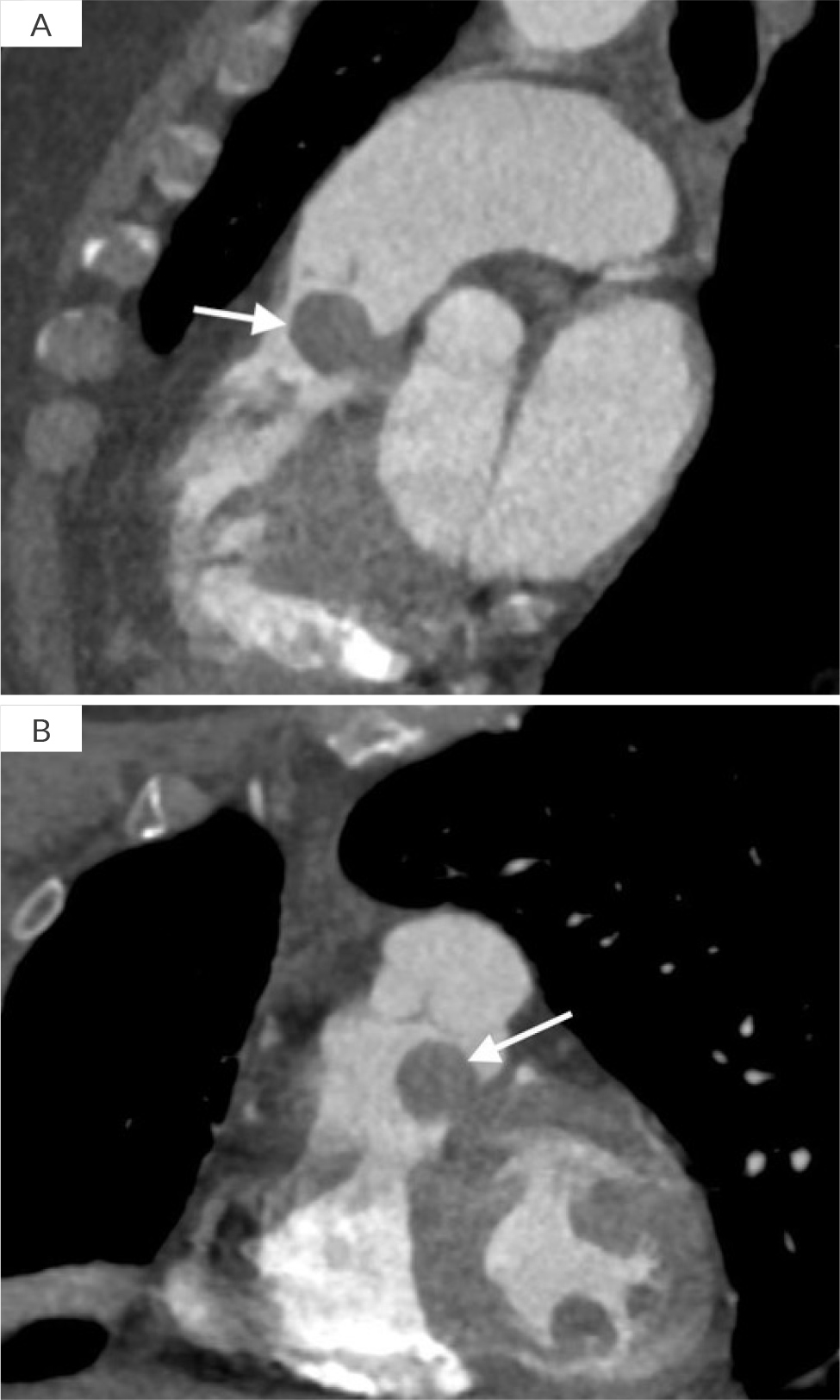
Initial blood work showed an elevated D-dimer level of 0.148 mg/l (reference range <0.0005 mg/l) and computed tomography angiography (CTA) of the chest ruled out pulmonary embolism. However, it revealed bibasilar infiltrates and a new 1.4 x 1.6 cm intracardiac heterogenous mass along the pulmonary valve (Fig. 1A and B). Acute COPD exacerbation with aspiration pneumonia was suspected and the patient was started on steroids, breathing treatments and antibiotics. Transthoracic echocardiogram (TTE) showed a well-circumscribed mass measuring 1.4 cm x 1.9 cm on the posterior leaflet of the pulmonary valve. The peak gradient across the pulmonary valve was estimated to be 25 mmHg, biventricular systolic function was normal. Right atrial pressure was 8 mmHg and peak tricuspid regurgitant velocity was 3.1 m/sec. Due to the presence of a mass in the right ventricular outflow tract (RVOT), estimated right ventricular systolic pressure (RVSP) (46 mmHg) is not equal to pulmonary artery (PA) systolic pressure. PA systolic pressure was 19 mmHg. A heparin drip was started for potential intracardiac thrombus, pending further evaluation. Meanwhile, the patient responded to the initial treatment and was extubated.
Figure 1. CT scan image showing a mass (white arrow) along the ventricular aspect of the pulmonary valve A) RVOT view and B) coronal view.
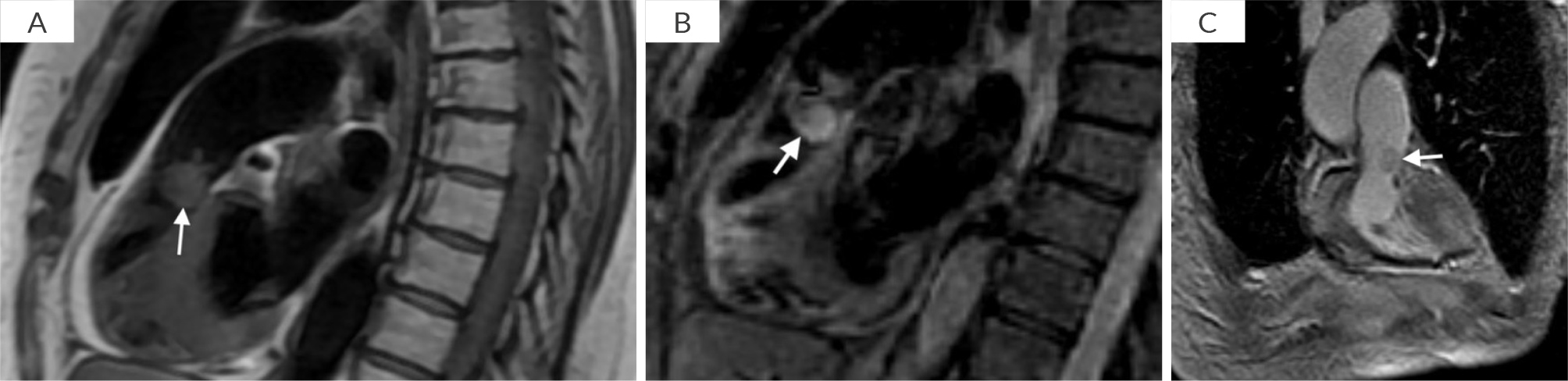
Cardiac magnetic resonance imaging (CMR) showed a large 1.3 x 1.5 cm mass on the ventricular side of the pulmonary valve with a stalk attaching it to the medial wall of the RVOT (Video 1A and B). The mass was not protruding into the main PA during systole. The mass was isointense on T1- weighted CMR and hyperintense on triple inversion T2-weighted CMR, suggesting vascularity (Fig. 2). Late gadolinium enhancement (LGE) CMR shows, patchy LGE in its core confirmed it to be a vascular tumor rather than a thrombus. The heparin drip was discontinued. A medical oncologist recommended biopsy with or without resection of the mass.
Video 1. CMR SSFP sequence demonstrating a mass along theventricular aspect of the pulmonary valve A) RVOT view and B) coronal view.
Figure 2. A) T1 weighted sequence, showing a isointense mass involving the pulmonary valve (white arrow); B) T2 weighted sequence, showing a hyperintense mass involving the pulmonary valve (white arrow); C) Pulmonary valve mass (white arrow) with patchy LGE in the core of the mass.
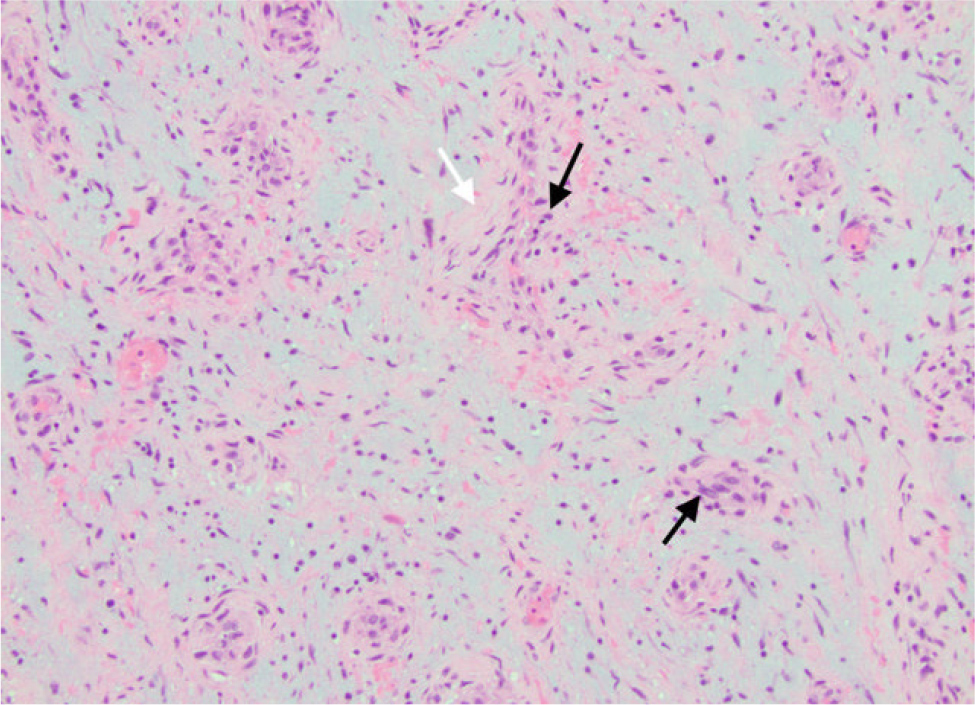
The patient was deemed to be at high surgical risk for open heart surgery. Conservative management was advised. A palliative team was involved in a detailed discussion of risks/benefits as well as prognosis of the disease. The patient declared that she understood the option of a palliative approach as well. However, she chose to proceed with a tissue biopsy to initiate/adjust chemo-/immunotherapy. Therefore, percutaneous removal of the mass was performed successfully under transesophageal echocardiographic (TEE) guidance using an AngioVac and FlowTriever catheter. She received a total of 4 mg intravenous (IV) ativan and 50 mcg of IV fentanyl during the procedure. Intraoperative findings are shown in video 2A and B. Histopathology of the mass revealed features suggesting myxoma with fibrosis, hemorrhage, and chronic inflammation (Fig. 3). The patient had an uneventful recovery and was discharged in stable condition.
Video 2. A) Intraoperative transesophageal echocardiogram showing a pulmonary valve mass and B) three-dimensional transesophageal acquisition of the pulmonary valve mass.
Figure 3. Histology slide of the surgical specimen showing spindle cells with eosinophilic cytoplasm (black arrows) and myxoid stroma (white arrow).
DISCUSSION
Cardiac myxoma is the most common (58-70%) benign primary cardiac tumor, commonly presenting in the third to sixth decades of life with a female predominance (female to male ratio of 2:1)[1-5]. About 75-80% of myxomas are known to originate in the left atrium, usually from interatrial septum near the fossa ovalis and less commonly in the right atrium (10-20%), or ventricles (6%)[5,6]. Myxomas originating from locations other than these typical locations are termed “atypical myxomas” as was the case in our patient.
The clinical presentation varies based on the location and extent of the disease. The presentation of myxoma can be categorized into 3 groups[2,3,7,8]:
- 1) Constitutional symptoms: fever, cough, fatigue, weight loss, loss of appetite, arthralgia, myalgia, Raynaud syndrome, and anemia.
- 2) Circulatory symptoms: symptoms related to obstruction of blood flow such as, syncope, dizziness, palpitations, shortness of breath, pulmonary edema, left/right heart failure.
- 3) Symptoms due to the potential of myxoma to embolize: stroke, myocardial infarction, and embolic limb ischemia, etc.
Symptoms of atypical myxoma are mostly from blood flow obstruction (90%) versus constitutional (5%) or from embolization (5%)[9]. Reportedly, atypical myxomas can arise from: the right atrium usually at interatrial septum, anterior or posterior walls of left or right atrium, near the inferior vena cava (even within the suprahepatic portion), within the superior vena cava, left ventricle, aortic valve, right ventricle[1-9]. They rarely have a multicentric or biatrial origin[7,8,9]. Cardiac myxoma originating from the pulmonary valve is relatively rare and the literature about it is very limited. Between 1950-2016, only 22 cases of pulmonary valve/PA myxomas were reported[10]. Most originated from the pulmonary valve leaflets, but involved the commissure, annulus, conus, or even the PA and the RVOT[10]. Those were typically smaller in size, with a propensity to cause right ventricular obstruction, right sided valvular insufficiency, pulmonary hypertension, and embolization to the PA. Circulatory and constitutional symptoms were common[10]. In our patient, the diagnosis of pulmonary valve myxoma was likely incidental as the sudden onset of symptoms and subsequent improvement with conservative management are not adequately explained by this tumor.
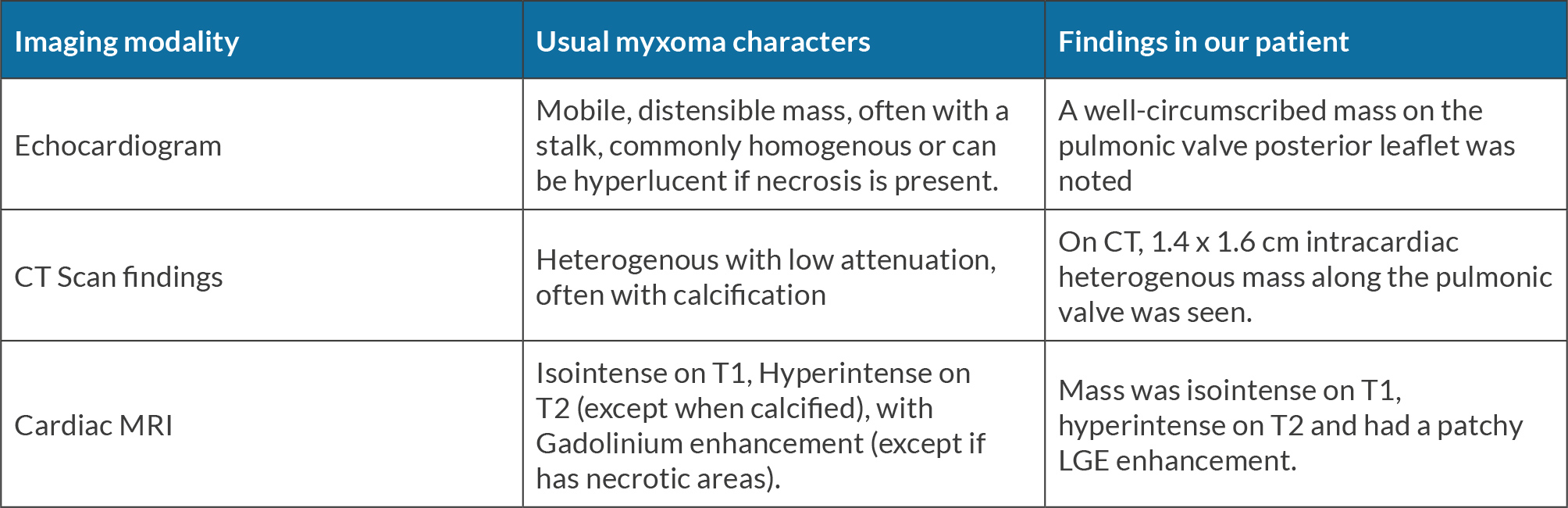
Atrial myxoma is diagnosed on echocardiography as a mobile, distensible mass with a characteristic narrow stalk, often prolapsing through the cardiac valves[2,3,6]. It can be homogenous or hyperlucent due to hemorrhage/necrosis. A broad-based and non-mobile myxoma is best characterized on CT scan or CMR. CT scan and CMR can also determine the extent of tumor infiltration into surrounding cardiac wall[3,6].
On CT scan, myxomas appear heterogeneous with low attenuation, often with calcification. Myxoma appears hyper-intense on T2-weighted CMR, however, calcification or hemosiderin may cause areas of decreased signal intensity. Intense contrast enhancement is often present unless there are necrotic areas within the tumor[6]. Myxoma involving the pulmonary valve or PA can cause filling defects in the main PA and/or RVOT on CT scan/CMR[10] (Table 1). Metastatic cardiac tumors can be heterogenous, with T2 hyperintensity and are often enhancing with gadolinium contrast. Imaging modalities can have a limited ability to distinguish myxoma from other tumors, (as in our patient). Histopathology examination after surgical resection can confirm the diagnosis. On gross examination, myxoma can be smooth or villous. Villous/papillary myxoma has a greater tendency to fragment spontaneously[2,3]. Microscopically, myxoma is characterized by small stellate to plump spindle cells with round, oval or elongated nuclei and abundant eosinophilic cytoplasm. The characteristic myxoid stroma is rich in mucopolysaccharides[2,3]. Hemorrhage and calcification can also be seen. Infrequently, glandular elements lined by columnar mucinous cells scattered in loose myxoid matrix are also noted in myxomas. Gross or histological features of typical and atypical myxomas do not differ significantly[9].
Table 1. Comparison of common myxoma characteristics with those found in our patient.
Recommended treatment is prompt surgical excision with removal of the attachment site of the tumor. This approach provides excellent early and long-term outcomes[3,11]. However, our patient was a poor surgical candidate, and the tumor was successfully removed percutaneously with intraoperative TEE using an AngioVac catheter and flow retrieval catheter. Despite clinical ambiguity due to the patient’s know history of pulmonary malignancy and the unusual location of the tumor, histopathology findings were consistent with cardiac myxoma. The percutaneous approach is associated with an increased risk of stroke and has been used in few cases previously. In the SEATTLE technique a Basket device and snare were used to remove right atrial myxoma[12]. Konecny et al. also proposed the use of a radiofrequency ablation catheter to remove a right-sided cardiac myxoma[13]. In the FLORIDA procedure an AngioVac catheter was used to remove a left atrial myxoma as in our case. Tanabe et al. described a case of asymptomatic pulmonary valve myxoma which was treated with resection and pulmonary valve replacement[14]. Roux et al. described a case of 16-year-old asymptomatic male with an incidental pulmonary valve mass which was diagnosed as myxoma based on histopathological features[15].
The patient profile and, hence, treatment options in that case were different from those in our case and CMR features were not described.
CONCLUSION
The minimally invasive treatment approach (removal of a tumor with intraoperative TEE using an Angioac and FlowTriever catheters) used to treat our patient appears to be effecxtive in high-risk surgical patients. Further studies are required to investigate this particular approach for pulmonary valve myxomas.