ABSTRACT
Hamman syndrome is defined as dissection of air in mediastinum and skin fascia usually due to increased intrathoracic pressure. The air leak tends to make its way into pleural and pericardial layers; however, in rare instances air can also dissect into epidural spaces, regarded as pneumorrhachis. We present a case of a young male with a history of polysubstance abuse and e-vaping, who presented with symptoms of altered mental status. Given the concerning physical examination, a computed tomography of the chest was undertaken, which showed pneumothorax, pneumomediastinum and pneumorrhachis. The patient was closely monitored in the intensive care unit and improved after symptomatic management. The symptoms of pneumorrhachis depend on the volume and location of air in intracranial and intraspinal space. Although asymptomatic in our case, it is crucial for clinicians to be aware that pneumorrhachis with Hamman syndrome can potentially cause neurological deficits and cardiopulmonary arrest in severe cases due to increased intraspinal and intracranial hypertension, emphasising the need for close monitoring.
KEYWORDS
Pneumorrhachis, hamman syndrome, e-vaping, polysubstance abuse
LEARNING POINTS
- Elevated intrathoracic pressure generated by deep inhalation of an aerosolised product is one of the triggers of air dissection in pleural, pericardial, and mediastinal regions. In rare instances, air can also translocate into intracranial and intraspinal spaces, which is referred to as pneumorrhachis.
- Mostly asymptomatic, pneumorrhachis has the potential to develop acute neurological deficits due to increased intracranial and intraspinal pressure, validating the need for acute monitoring.
- Most cases of pneumorrhachis are managed conservatively. However, severe cases warrant decompression or high concentrations of oxygen administration to facilitate air absorption.
INTRODUCTION
Vaping, regarded as a less hazardous substitute for smoking, entails the inhalation of vaporised substances via electronic cigarettes. Although vaping may seem harmless it carries potential risks, including infrequent yet severe conditions such as pneumomediastinum and pneumorrhachis. Pneumorrhachis is the presence of air inside the spinal canal. This rare medical condition is caused by the entry of air into the subarachnoid or epidural space that envelops the spinal cord. Pneumorrhachis may arise due to a multitude of factors: encompassing infections, surgical interventions or trauma. Injuries involving blunt force trauma, vertebral fractures or penetrating trauma have the potential to introduce air into the spinal canal. Medical procedures that involve the thoracic or cervical regions may be iatrogenic[1-4]. Acute complications may result from pneumorrhachis, including spinal cord compression and neurological deficits, although the condition is generally asymptomatic. Often, an imaging modality such as a computed tomography (CT) scan is utilised to diagnose the condition[5]. The approach to management is contingent upon the root cause and possible complications. Pneumorrhachis is a topic of interest for medical experts given its rarity; thorough knowledge is necessary for an accurate diagnosis and suitable treatment. Primarily arising after trauma, we describe a non-traumatic case of pneumorrhachis where the patient developed pneumorrhachis, pneumomediastinum and pneumothorax due to vaping.
CASE DESCRIPTION
A 29-year-old male was brought to the hospital by the police after they found him wandering in a car park. On arrival the patient was confused, mildly tremulous and had slurred speech. The patient did not have a memory of his recent whereabouts. Given the patient’s presentation, most of the history was obtained via prior electronic health records. His past medical history included anxiety, depression, polysubstance abuse and benzodiazepine overdose. He also had a history of e-vaping for several years. His vital signs on presentation were also unremarkable. He was saturating 98% of the room air without any obvious sign of distress.
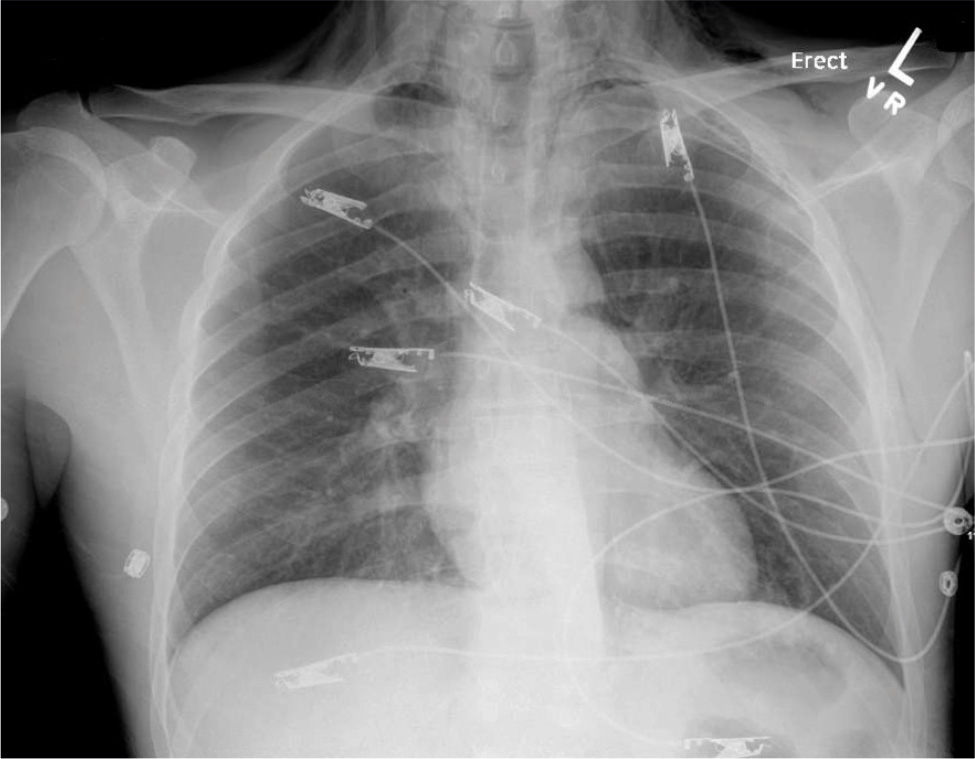
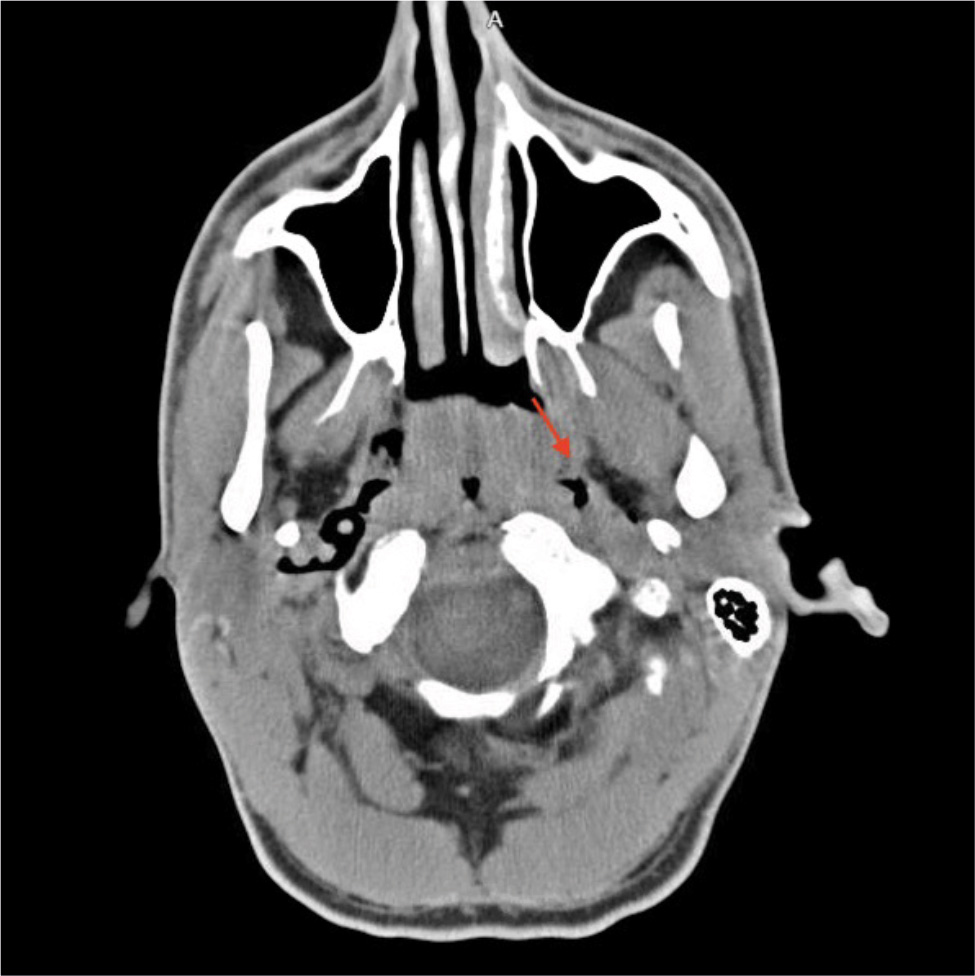
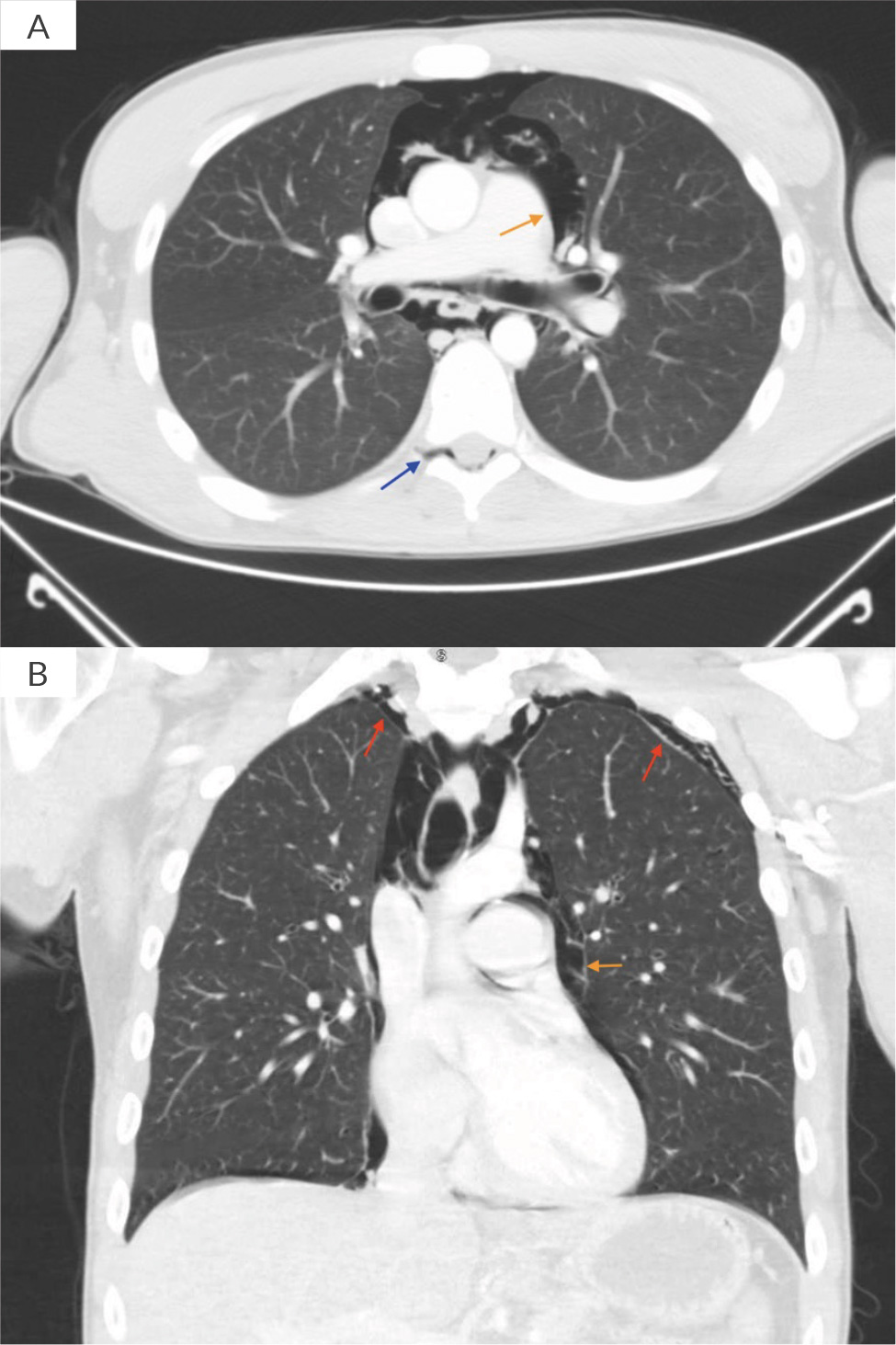
On physical examination, there were no signs of obvious trauma. His face was flushed, and palpable crepitus was appreciable on his neck and chest. Pulmonary examination was positive for auscultatory crepitations without any wheezes or crackles. The rest of the examination including cardiovascular, neurological, abdominal and musculoskeletal tests remained unremarkable. Initial laboratory results were significant for a white blood cell count of 25.4 × 103/µl, blood urea nitrogen 36 mg/dl and creatinine 1.31 mg/dl. Troponin was negative. Urine toxicology was positive for benzodiazepines, opioids and cannabinoids; urinalysis was also normal. Blood cultures were also obtained. A chest X-ray was performed which showed subcutaneous emphysema, pneumothorax and pneumomediastinum. No rib fracture was observed (Fig. 1). A CT scan of the head without contrast was performed for altered mental status which did not show any cranial pathology; however, it showed emphysema involving mostly the retropharyngeal and parapharyngeal spaces (Fig. 2). This was followed by a CT chest scan, which showed extensive pneumomediastinum, bilateral pneumothoraces and small-volume pneumorrhachis (Fig. 3).
Figure 1. Chest X-ray showing pneumothorax, pneumomediastinum and subcutaneous emphysema.
Figure 2. Emphysema involving mostly the retropharyngeal and parapharyngeal spaces.
Figure 3. CT chest scan with contrast showing pneumomediastinum (brown arrows), and evidence of pneumorrhachis (blue arrows) and pneumothorax (red arrows).
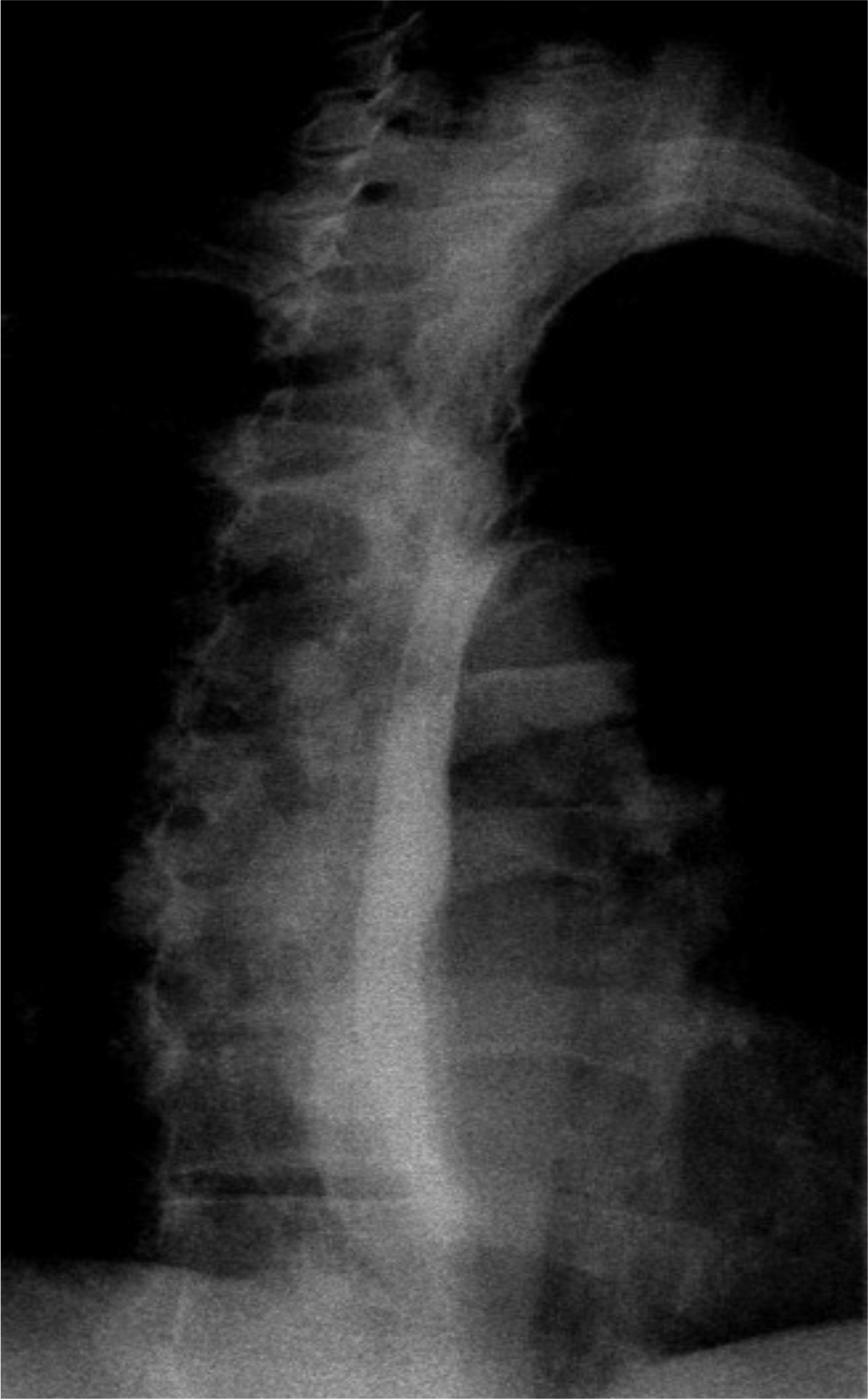
Due to concerns of impending respiratory failure, neurological deficits and suspicion of oesophageal rupture, the patient was admitted to the ICU for close monitoring. The patient was started on empiric antibiotics and an antifungal regimen. Meanwhile, the patient continued to remain agitated and was started on scheduled doses of olanzapine. Cardiothoracic surgery was consulted and an oesophagram was planned once mental status improved enough to swallow barium contrast. Over the period of 24 hours, the patient’s leukocytosis started to resolve, and respiratory status continued to remain stable. Serum creatinine level also improved. On hospital day 3, the patient’s mental status mildly improved. A fluoroscopic oesophagogram was performed which did not reveal oesophageal contrast leak, eliminating Boerhaave syndrome (Fig. 4). The antibiotics were discontinued, and the patient was downgraded to medical floors for further care until his mental status completely resolves. The patient was eventually sent to a rehabilitation centre for recovery.
Figure 4. Fluoroscopic oesophagogram showing normal contrast transit through oesophagus without any evidence of leak.
DISCUSSION
In 1939, Hamman first described spontaneous mediastinal and subcutaneous emphysema usually without any underlying lung pathology[1]. It is pathophysiologically explained by the dissection of air in bronchovascular sheath after alveolar rupture following elevated intra-alveolar pressure, low peri-alveolar pressure or a combination of both[2]. This phenomenon is known as the Macklin effect, often predisposed by triggers like Valsalva manoeuvres, violent coughing, vomiting, barotrauma, foreign body aspiration, etc. The use of e-cigarettes, vaping and inhalation of other illicit drugs including marijuana and cocaine have also been reported in literature. Deep inhalation increases large amounts of aerosolised product which are followed by prolonged exhalation against closed glottis which improves the absorption of the smoked product, enhances the euphoria effect, increases intrathoracic pressure and leads to higher chances of alveolar rupture causing pneumothorax[3,4,6]. Air can track its way through tissue planes and pleural layers, leading to the development of subcutaneous emphysema, pneumomediastinum, pneumopericardium and in rare instances, pneumorrhachis – defined as free air in the spinal cord. The positive pressure air especially in the posterior mediastinum drives air through neural foramina into epidural space, which lacks fascial layer protection[7]. Similar to our patient, reports of pneumorrhachis as a consequence of marijuana, cocaine and MDMA (3,4 methylenedioxymethamphetamine) ‘ecstasy’ smoking have also been reported[8]. The most common presenting symptoms of pneumomediastinum include chest pain (around 70% of the patients) followed by shortness of breath, cough, neck pain, odynophagia, and voice hoarseness[4,8]. Around 60% of the patients also have auscultatory or palpable crepitus or Hamman’s sign over the mediastinum and neck, indicative of subcutaneous emphysema[9].
Pneumorrhachis is usually asymptomatic especially if it is epidural in nature (external pneumorrhachis) like in our patient. However, internal pneumorrhachis – air located in subdural or subarachnoid space – usually develops as a complication of penetrating injury, spinal infection by gas-forming bacteria or lumbar puncture and has the propensity to extend centrally causing pneumocephalus. It is important to note that pneumocephalus can present with a wide array of symptoms including headache, vomiting, seizures, hemodynamic instability and in severe cases, cardiopulmonary arrest. The spectrum of symptoms depends upon the volume, location and tension of air trapped in an intracranial compartment exacerbating intracranial and intraspinal pressure[5,10]. Due to the compactness of central nervous tissues, an air collection of even less than 2 ml in subarachnoid space is enough to produce a headache[10]. Due to the absence of corresponding findings in the CT scan of the head of our patient, the altered mental status was explained well by illicit drug overdose.
In 1983, ultrasonographic evaluation of pneumomediastinum was first described as the ‘air gap sign’, defined as bands of echoes reflecting accumulated air shadowing normal cardiac structures, which have a cyclical appearance with each cardiac cycle. The differentiation of pneumomediastinum from pneumopericardium on point-of-care ultrasonography (PoCUS) depends on the angle of view. The clear visualisation of the heart in the parasternal and apical view along the subxiphoid view is indicative of pneumomediastinum. In the pneumopericardium, the heart is not visible in the subxiphoid view as air extends inferiorly to the posterior reflection of the pericardium and disperses sound waves[11]. However, in our patient, PoCUS was not performed bedside as the patient did not have respiratory distress or other concerning symptoms on presentation.
Although a chest X-ray is able to define air collection in the pericardium, pleural space, subcutaneous tissues or mediastinum, a CT scan of the chest is the choice of modality, given high sensitivity to identify air in extra pneumatic spaces, especially the spinal cord. Pneumorrhachis is usually observed in the posterior epidural space due to the presence of loose connective tissue as compared to a dense vascular network in the anterior zone[4,5]. Exclusion of air leaks from the aerodigestive tract through esophagoscopy and bronchoscopy is also crucial in suspected cases[2]. As most of the cases of pneumomediastinum and pneumorrhachis are uncomplicated with mild or no symptoms, supportive care (bedrest, oxygen supplementation, analgesia) remains the initial approach[12]. Other treatment modalities for pneumorrhachis include intravenous dexamethasone, decompression of the epidural space with Tuohy needle and high concentration oxygen therapy with the idea of increasing absorption of air from through subdural space. Regardless, patients need close monitoring to avoid potentially serious complications like respiratory or neurological compromise[12]. It is crucial to note that the aerosol used in e-vaping is not only nicotine but other substances such as cannabinoids. Several cases have been reported in the literature which cause pneumomediastinum and are often related to chest pain. However, these cases were associated with chest pain which was not reported by our patient given his delirious state[4].
CONCLUSION
This case report underscores an atypical, symptomless presentation of pneumorrhachis, thereby drawing attention to the difficulties in diagnosis and the necessity for personalised interventions when symptoms are absent. In patients presenting with pneumothoraces and a history of e-cigarette use, it is imperative to consider the possibility of pneumorrhachis due to the heightened risk of complications. This contributes to the comprehension of various clinical manifestations by demonstrating the critical significance of advanced imaging modalities, including CT scans and MRIs, in the characterisation of pneumorrhachis.