ABSTRACT
Background: Cardiac sarcoidosis can cause a wide range of symptoms, including shortness of breath, chest pain, oedema, and fatal arrhythmias such as ventricular tachycardia (VT). Because the symptoms can be nonspecific, diagnosing cardiac sarcoidosis can be challenging. Treatment options may include corticosteroids to reduce inflammation, immunosuppressive drugs to prevent further damage, medications to control symptoms, ablation procedures, and defibrillators to prevent cardiac arrest.
Case: A 60-year-old woman who has sarcoidosis affecting multiple organs including cardiac sarcoidosis, non-ischemic cardiomyopathy with reduced ejection fraction, and hypertension, was admitted with tachycardia, shortness of breath, and a recently fired automatic implantable cardioverter defibrillator (AICD). Three months prior, the patient was admitted for a syncopal episode and diagnosed with cardiac sarcoidosis through cardiac magnetic resonance imaging (MRI) and positron emission tomography (PET), which demonstrated active inflammation, and an AICD was implanted. During this admission, the patient had an episode of ventricular tachycardia and was treated with amiodarone and lidocaine. The patient received steroids, sacubitril/valsartan, and methotrexate. After 48 hours of observation, the patient was discharged without further events.
Conclusion: Cardiac sarcoidosis is a rare but serious disease that can lead to life-threatening cardiac complications such as ventricular tachycardia. Early diagnosis and aggressive management are crucial for improving outcomes and preventing sudden cardiac death. AICD implantation as a secondary prevention in cardiac sarcoidosis might prevent cardiac arrest.”
KEYWORDS
Sarcoidosis, arrhythmia, ventricular tachycardia
LEARNING POINTS
- Cardiac sarcoidosis can present with non-specific symptoms and lead to life-threatening arrhythmias such as ventricular tachycardia, emphasising the importance of early diagnosis and aggressive management to prevent sudden cardiac death.
- A multidisciplinary approach involving imaging modalities such as cardiac magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, along with histological findings, is crucial for accurately diagnosing cardiac sarcoidosis, as endomyocardial biopsy alone has low sensitivity.
- Implantation of an automatic implantable cardioverter defibrillator (AICD) as a secondary prevention measure should be considered in cardiac sarcoidosis patients, even in elderly individuals with mildly to moderately reduced ejection fraction, to prevent fatal arrhythmias and sudden cardiac death.
INTRODUCTION
Sarcoidosis is a granulomatous disease whose prevalence varies greatly depending on the region of the world. In one recent study where the prevalence of sarcoidosis was evaluated, the prevalence ranged from 2–160 per 100,000[1]. In patients with sarcoidosis, the heart is involved in 5–25% of the cases[2]. The rates of ventricular arrhythmias and cardiac arrest are higher in patients with cardiac sarcoidosis compared to the general population, as seen in one recent study in which 18,013,878 patients were evaluated, of which 46,289 had sarcoidosis[3].
Ventricular tachycardia can be the sole manifestation of sarcoidosis. A case report by Yasir et al. discussed a 27-year-old female with no past medical history presenting with recurrent ventricular tachycardia[4]. The incidence of adverse events such as cardiac arrest, arrhythmias and progressive heart failure symptoms increases as the clinical features of cardiac sarcoidosis become more apparent. Sarcoidosis is a diagnosis of exclusion and the triad used for diagnosis includes 1) compatible clinical or radiological findings of sarcoidosis; 2) histological findings suggestive of sarcoidosis (non-caseating granulomas); and 3) exclusion of other granulomatous diseases.
However, the diagnosis of cardiac sarcoidosis is more challenging partly because of the low sensitivity of the endomyocardial biopsy (36%)[5]. The low sensitivity of the endomyocardial biopsy can be explained by the patchy involvement of the myocardium. The addition of another histological finding, increased lymphatic vessel counts on the myocardial biopsy of patients with cardiac sarcoidosis, increases the sensitivity to 75% even in the absence of a non-caseating granuloma[5]. The addition of radiological modalities such as an echocardiogram, cardiac magnetic resonance imaging (MRI) and a fludeoxyglucose-18 positron emission tomography (FDG-PET) scan help physicians in diagnosing cardiac sarcoidosis. The echocardiogram findings are non-specific but can include interventricular septal thinning (primarily basal), which is most typical for cardiac sarcoidosis[6]. The cardiac MRI usually shows late gadolinium enhancement, particularly in the basal segments, epicardium and mid-myocardium[6]. The FDG-PET scan can guide clinicians regarding disease activity, and focal and diffuse-on focal FDG uptake and suggests active cardiac sarcoidosis.
When considering FDG-PET scans, it is essential to differentiate between conventional PET scans and cardiac-specific PET scans, especially in the context of sarcoidosis. Cardiac PET scans require specific patient preparation to enhance diagnostic accuracy, including dietary restrictions and glucose control, which are crucial for reducing physiological myocardial glucose uptake and improving the detection of pathological FDG uptake indicative of inflammation or active sarcoidosis. This preparation contrasts with the general preparation for conventional PET scans, emphasising the need for tailored protocols when assessing cardiac involvement in sarcoidosis[7].
Three major guidelines exist that help aid clinicians in diagnosing cardiac sarcoidosis. These guidelines are as follows: 1) WASOG sarcoidosis organ assessment instrument; 2) the HRS expert consensus statement; 3) the Japanese Ministry of Health and Welfare guidelines[5]. The most common presentation of cardiac sarcoidosis is arrhythmia, and the prevalence of specific arrhythmias is as follows: 26–62% atrioventricular block, 2–42% ventricular tachycardia (VT) and 12–65% sudden cardiac death[3].
CASE DESCRIPTION
A 60-year-old African American female presented with tachycardia, shortness of breath and a fired, recently placed implantable cardioverter defibrillator (AICD). She had a history of sarcoidosis confirmed with skin biopsy showing the presence of non-caseating granuloma, including cardiac sarcoidosis, pulmonary sarcoidosis with bilateral hilar adenopathy and cutaneous sarcoidosis, with erythema nodosum on her lower extremities.
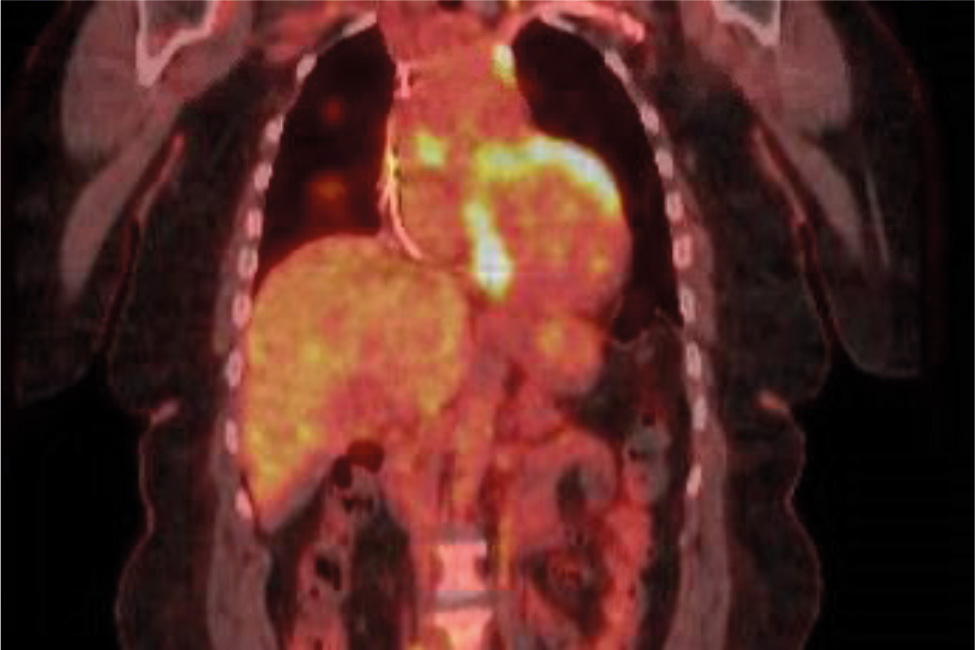
Over the past 5 years, the patient’s sarcoidosis had been managed with oral prednisone 20 mg daily. Her pulmonary and cutaneous manifestations remained stable on this regimen. However, 3 months prior to this admission, the patient was admitted for a syncopal episode and intermittent nausea. An electrocardiogram (EKG) at that time revealed a new onset first-degree atrioventricular block. Subsequently, the patient underwent a left heart catheterisation which showed normal coronary arteries, and a transthoracic echocardiogram (TTE) scan, which revealed a reduced ejection fraction of 45%. A cardiac MRI scan was consistent with cardiac sarcoidosis (Fig. 1), and a PET scan demonstrated active inflammation. An AICD was placed for secondary prevention before discharge.
Figure 1. Cardiac MRI showing active inflammation in the setting of cardiac sarcoidosis.
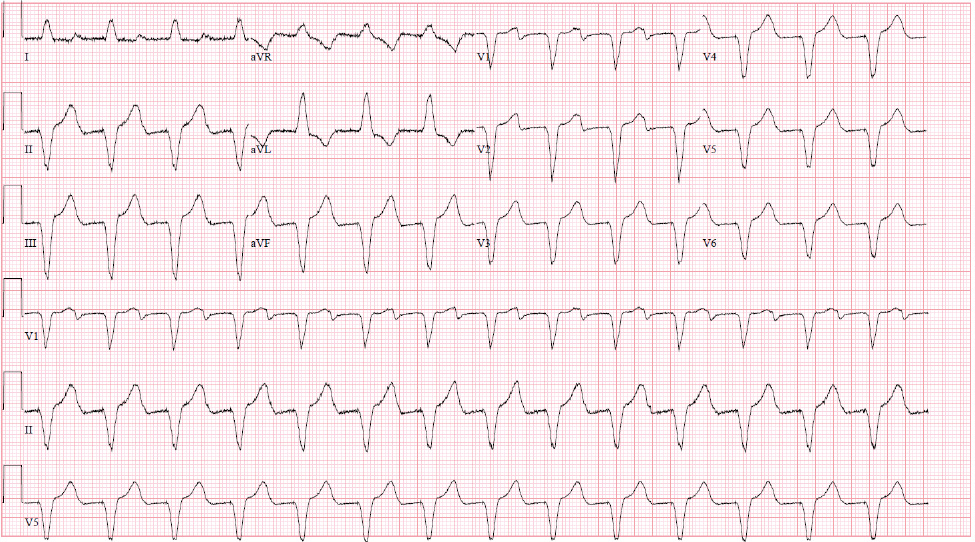
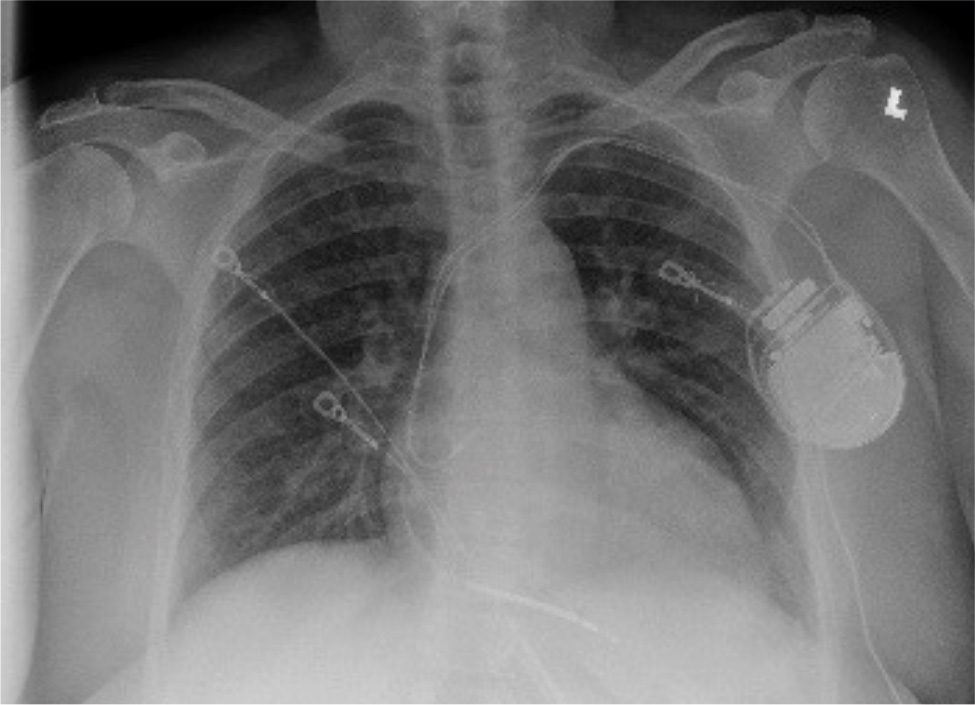
On this admission, the patient was in mild distress, hypertensive with BP 160/90 mmHg, non-tachycardic and afebrile. Laboratory values were only notable for an elevated serum pro-brain natriuretic peptide of 1,273; the troponin level was within normal limits. An EKG (Fig. 2) revealed a ventricular-paced rhythm and an X-ray (Fig. 3) of the chest revealed bilateral interstitial and nodular opacities, most prominent at the retrocardiac region, which was consistent with known sarcoidosis. A repeated TTE during this admission showed increased left ventricular wall thickening and a reduced ejection fraction of 48%. The ICD was interrogated on admission and showed an episode of VT with an ICD discharge.
Figure 3. Bilateral interstitial and nodular opacities, most prominent at the retrocardiac region, which was consistent with known sarcoidosis, with the presence of an AICD.
While in the emergency department, the patient had an episode of VT refractory to an unsuccessful external shock, and multiple unsuccessful ICD shocks. Successful conversion was achieved after intravenous lidocaine and amiodarone. The patient was haemodynamically stable throughout this episode; she was started on steroids and subsequently admitted to the cardiac care unit (CCU) for further management.
In the CCU the patient was treated with amiodarone, metoprolol, sacubitril/valsartan and steroids, and started on methotrexate. After observation in the CCU for 48 hours, the patient was transferred to the telemetry floor and subsequently discharged after no events on the telemetry monitor for more than 48 hours.
After discharge, the patient continued on her immunosuppressive regimen with prednisone 20 mg daily and methotrexate 10 mg once a week for her cardiac sarcoidosis and was followed closely by her pulmonologist and cardiologist. She also remained on amiodarone and metoprolol for management of her VT. At her 6-month follow-up visit with cardiology, interrogation of her AICD showed normal device function, adequate battery life and no new VT events since the initial episode. The patient was scheduled to follow up with pulmonology the following month. She was enrolled in remote monitoring for her AICD and scheduled for a return visit with cardiology in 6 months to reassess her cardiac status.
DISCUSSION
Cardiac sarcoidosis can lead to devastating outcomes including sudden cardiac arrest caused by a wide array of arrhythmias. To manage cardiac sarcoidosis effectively, clinicians employ a dual strategy focusing on the disease itself with immunosuppressive therapy and addressing the cardiac manifestations with appropriate medical and interventional treatments. This comprehensive approach includes managing heart failure, correcting conduction abnormalities with anti-arrhythmic medications and in some cases, considering more advanced interventions such as pacemakers, implantable cardiac defibrillators or cardiac transplant[6,8].
The treatment of sarcoidosis with immunosuppressants is a nuanced approach, particularly when standard therapies such as corticosteroids are insufficient or cause significant side effects. Infliximab, an immunosuppressive biologic, has been identified as an effective treatment option for refractory sarcoidosis, especially in cases involving the heart and other severe manifestations[9]. Different studies suggest that the implantation of a defibrillator is preferred in young patients with low ejection fraction[10]. Other studies recommend a cut-off ejection fraction (EF) of 35% for placement of a cardiac defibrillator[11]. In our case the patient was 60 years old with a moderately reduced EF of 45%, who received an AICD as a secondary prevention of cardiac arrest secondary to arrhythmias. Our case report explores the consideration of an AICD implantation in elderly patients with mild to moderately reduced EF.
CONCLUSION
Cardiac sarcoidosis can lead to dangerous arrythmias resulting in sudden cardiac death. The diagnosis of cardiac sarcoidosis early on is imperative as morbidity and mortality of the disease is improved significantly. Therapies including immunosuppressive treatment, catheter ablation, complication directed medical therapy and AICD implantation as a secondary prevention of sudden cardiac death should be explored.