ABSTRACT
Introduction: We present a case of anion gap euglycemic diabetic ketoacidosis (EuDKA) in a patient with COVID-19 infection. Patients with diabetes mellitus are at increased risk of severe illness, and hyperglycaemia is associated with higher morbidity and mortality in patients infected with COVID-19.
Case Description: A 76-year-old male with diabetes mellitus treated with SGLT2 inhibitor tested positive for COVID-19 infection on day 3 after his admission. In the emergency room he had a high anion gap metabolic acidosis and a blood glucose of 248 mg/dl. His urine tested strongly positive for ketones. A diagnosis of euglycemic diabetic ketoacidosis was made and he was treated with intravenous insulin and normal saline; his antidiabetic medications were stopped. His metabolic acidosis gradually resolved, and he was discharged.
Discussion: Euglycemic diabetic ketoacidosis is a rare complication of COVID-19 infection. It is defined by the American Diabetes Association as the triad of anion gap metabolic acidosis with arterial pH <7.3, serum bicarbonate <18 mmol/l and ketonuria or ketonemia. It is a life-threatening complication which usually occurs in type 1 diabetes mellitus patients but may also occur in type 2 diabetes mellitus patients. As described earlier, it is associated with hyperglycaemia but if blood glucose is low or near normal but <250 mg/dl it is then named euglycemic diabetic ketoacidosis. Patients treated with SGLT2 inhibitors are at increased risk of euglycemic diabetic ketoacidosis.
Conclusions: COVID-19 infection precipitated euglycemic diabetic ketoacidosis in our patient. SGLT2 inhibitors must be stopped when this adverse reaction occurs. As their use increases, the risk of this adverse reaction is higher as well. Their prescription should be restricted to trained physicians who are able to educate their patients and treat them appropriately in situations that may arise.
KEYWORDS
COVID-19, diabetic ketoacidosis, euglycemic, SGLT-2 inhibitors
LEARNING POINTS
- COVID-19 infected patients are at increased risk of developing diabetic ketoacidosis or euglycemic ketoacidosis when treated with SGLT-2 inhibitors.
- It is practical to discontinue the drug at the onset of any symptoms consistent with acute infection to prevent the development of euglycemic diabetic ketoacidosis.
INTRODUCTION
COVID-19 infection is a cause of diabetic ketoacidosis as it can lead to ketogenesis through many pathways[1]. Euglycemic ketoacidosis is defined by the American Diabetes Association as the triad of anion gap metabolic acidosis with arterial pH <7.3, serum bicarbonate <18 mmol/l, and ketonuria or ketonemia. It occurs more commonly in patients with type 1 diabetes mellitus as a result of suboptimal insulin treatment but may also occur in patients with type 2 diabetes mellitus. Blood glucose may be 200–250 mg/dl but it could be <200 mg/dl or even normal in euglycemic diabetic ketoacidosis; this may delay the diagnosis. A meta-analysis of 36 trials for type 2 diabetes mellitus patients demonstrated an increased risk of diabetic ketoacidosis in those taking SGLT2 inhibitors (OR 2.07, 95% CI 1.44–2.98) versus other therapies[2]. A similar high risk for diabetic ketoacidosis (DKA) was demonstrated in a population-based cohort study from Canada and the UK[3]. Canagliflozin was associated with the higher risk among the three SGLT2 inhibitors[3].
Serum ketones should be measured in patients taking SGLT2 inhibitors with nausea, vomiting or malaise, and if acidosis is eventually confirmed, SGLT2 inhibitors should be stopped. Because the risk of DKA was demonstrated in all trials with type 1 diabetes mellitus patients, their off-label use should be discouraged.
CASE DESCRIPTION
A 76-year-old man with personal history of hypertension, type 2 diabetes mellitus and dyslipidaemia presented to the emergency department complaining of fever and multiple episodes of vomiting over the previous 24 hours. He also complained of fatigue and malaise. An initial test for COVID-19 performed by his primary care physician was reported to be negative.
Physical examination in the emergency department was reported as unremarkable. He had a body weight of 80 kg, a height of 170 cm and thus a BMI of 27.7 kg/m2, and initially oxygen saturation was 95% on ambient air. Arterial blood gases showed a metabolic acidosis with an elevated anion gap (pH 7.07, paCO2 15 mmHg, paO2 92 mmHg, HCO3 7.7 mmol/l, BE -25.5 mmol/l, anion gap 29.3 mEq/l, lactate 2.2 mmol/l) and his blood glucose was 248 mg/dl. He had a mildly elevated urea of 56 mg/dl and creatinine was 1.44 mg/dl. His electrolytes were within normal range. His urine tested strongly positive for ketones and a diagnosis of euglycemic diabetic ketoacidosis was made. He was admitted and started on intravenous insulin and normal saline. He also received intravenous dextrose when required, according to the protocol of DKA treatment. All his oral antidiabetic medications were stopped.
Fever, nausea, and vomiting persisted in the subsequent three days, and he developed dyspnoea. His SARS-CoV-2 RT-PCR test was repeated on the third day, and it was positive. Oxygen saturation dropped to 88% on ambient air and on examination, he had right basal crackles on chest auscultation but no wheeze or rales.
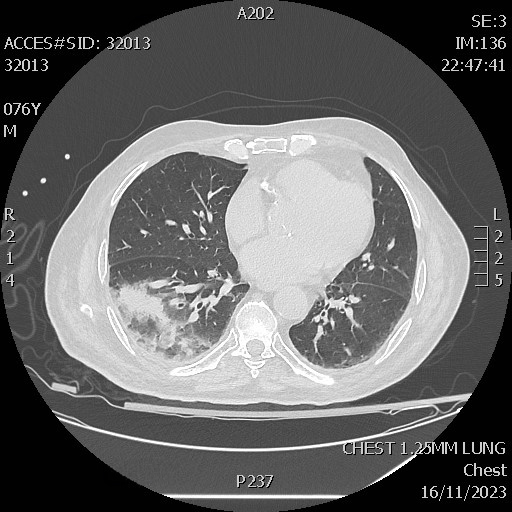
His chest radiograph was reported as normal but a CT chest scan that followed showed right basal confluent ground glass infiltration (Fig. 1). Interleukin 6 was found to be 22 pg/ml (normal range <7 pg/ml).
After beginning intravenous fluids and insulin, the patient recovered with gradual improvement of his acidosis; his HbA1c was 9%. He was discharged 10 days later in very good clinical condition with resolution of his presenting symptoms.
DISCUSSION
Euglycemic DKA has been defined as hyperglycaemia with glucose <250 mg/dl. SGLT2 inhibitors have been associated with euDKA as it occurs more frequently in patients treated with SGLT2 inhibitors than in non-users.
Common precipitating factors of euDKA are acute illnesses (infections, vomiting, abscesses, fractures) followed by insulin reduction or cessation, and low BMI, alcoholism, low adherence to medication and a low carbohydrate diet[4]. COVID-19 infection acts as a precipitating factor for DKA and increases further the risk in patients treated with SGLT2 inhibitors.
SGLT2 inhibitors increase renal clearance of glucose and lead to low blood glucose in the setting of acute illness. The reduced insulin requirements in combination with increased insulin resistance will lead to ketosis and euDKA. Vomiting, reduced food intake and insulin doses are precipitating factors for DKA. Hepatic glucose production reflected by fasting plasma glucose is inhibited by low portal insulin concentrations. Higher insulin would be required to suppress ketone production[5-7]. Fasting plasma glucose can be reasonable despite very low portal insulin levels, as glucose is lost in urine. This increases susceptibility to ketosis and may disconnect ketosis from hyperglycaemia. Use of SGLT2 inhibitors is also associated with hyperglucagonaemia but the mechanism has not been elucidated. High glucagon levels increase the risk of ketone production, and they can also worsen a hypovolaemic condition through their mechanism of action. Increased glucagon/insulin ratio and increased cortisol and epinephrine are found in patients with hypovolaemia and these further increase insulin resistance, lipolysis and ketogenesis[6-8].
Pancreatic islet cell death may be increased in patients with COVID-19 infections due to increased expression of angiotensin converting enzyme 2 receptors[6,9]. Interleukin-6 is also found to be increased in COVID-19 patients and this elevation is even higher in patients with impaired glucose tolerance[6,10].
Therefore, a cytokine storm is another precipitating mechanism of euglycemic DKA in patients with COVID-19 infection treated with SGLT2 inhibitors[6,10].
Patients with type 1 or type 2 diabetes mellitus treated with SGLT2 inhibitors should be evaluated for DKA if they develop nausea, vomiting or malaise, even if glucose levels are relatively normal. They should have their urine and/or plasma ketones checked either at home or in the hospital. If euglycemic DKA is detected then it can be prevented and alleviated by maintaining fluid and carbohydrate intake, and by stopping SGLT2 inhibitors[7].
Given the frequency of euDKA we should be aware of this complication especially for patients with type 2 diabetes mellitus who have personal history of DKA who are treated with SGLT2 inhibitors, and especially for those who are in the perioperative period as the duration of their metabolic effects is not known[5].
All patients treated with SGLT2 inhibitors who become acutely unwell or if the carbohydrate intake is insufficient, should stop taking them as they make them prone to euglycemic diabetic ketoacidosis[9]. They should also be aware of the possibility of developing diabetic ketoacidosis and the precipitating factors and be advised to seek medical care when they develop symptoms of ketoacidosis.
Although SGLT2 inhibitors have a half-life of around 12h, the pharmacodynamic effects could last longer and simply stopping the drug 24–48h before surgery or when they become acutely unwell may not be effective. Research in the future may clarify the duration of their metabolic effects so that appropriate guidance is provided to prevent euglycemic diabetic ketoacidosis[5].