ABSTRACT
We describe a rare case of polyserositis with chylous ascites following nivolumab therapy, highlighting the challenges in recognising and managing immune-related adverse events (irAEs) associated with immune checkpoint inhibitors (ICPIs).
Induced polyserositis and chylous ascites are very rare and require immunosuppressive treatment, with a variable response of high-dose IV steroids.
KEYWORDS
Immune-related adverse events, cancer, immune checkpoint inhibitors, immunotherapy
LEARNING POINTS
- Oncological therapy with immune checkpoint inhibitors (ICPIs) is frequently associated with immune-related adverse events (irAEs) most involving cutaneous, gastrointestinal and pulmonary sites.
INTRODUCTION
Immune checkpoint inhibitors (ICPIs) enhance immune system identification and the destruction of tumour cells. Thus, they are used in several tumour types such as melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma, hepatocellular carcinoma and Hodgkin lymphoma[1].
Among them, nivolumab is a fully human IgG4 that selectively blocks the interaction of the programmed death-1 (PD-1) receptor with its ligands, disrupting signals that downmodulate T-cell activation and proliferation. This action is often related to immune-related adverse events (irAEs) involving different organs, especially gastrointestinal, cutaneous and hepatic sites; serositis with pericardial, pleural and/or peritoneal effusions are also described in case reports and series[2,3]. These kinds of adverse events usually respond to drug withdrawal and immunosuppression with glucocorticoids; alternatively, immune modulating agents including mycophenolate mofetil, infliximab, anti-interleukin-6 agents or intravenous immunoglobulin have been used, but prospective studies on their safety and efficacy are still lacking[2].
CASE DESCRIPTION
In 2016, a 39-year-old man was diagnosed with a stage IIIC melanoma (T3aN2M0) with V600E BRAF mutation located on his right leg. He underwent partial right inguinal lymphadenectomy and subsequently received adjuvant therapy with pembrolizumab, leading to a complete remission of the disease. Due to relapse of the disease with metastases to lymph nodes and colon, in February 2022 he was treated with encorafenib and binimetinib for 12 weeks, then nivolumab plus ipilimumab for 48 weeks. At the end of the induction treatment, the patient achieved complete remission. Therefore, in October 2022 he started maintenance therapy with nivolumab at a dosage of 480 mg every 4 weeks. Levothyroxine 100 mcg qd and edoxaban 60 mg qd were added due to subclinical hypothyroidism (TSH 9.17 mcU/ml, FT4 1.31 ng/dl) and right proximal saphenous vein thrombosis.
In November 2022 (6 weeks after the first infusion) he developed fever and periorbital oedema, and the therapy was suspended. After two more weeks, the patient presented at the emergency department with malaise and dyspnoea. A total body computed tomography (CT) scan showed pleural and pericardial effusion and ascites, with no findings suggesting a recurrence of melanoma. He was admitted to our Internal Medicine Ward in December 2022. Microbiology and autoimmunity panels returned negative results. Echocardiography showed normal findings except for a small pericardial effusion without haemodynamic impact. Nephrotic syndrome was ruled out, while the size of the pleural, pericardial and abdominal effusions was not conducive to invasive procedures. The patient received treatment with furosemide and intravenous methylprednisolone at a dosage of 1 mg/kg for five days. Monitoring through echocardiography documented a reduction in the size of the effusions, following which the patient transitioned to oral prednisone at a dose of 1 mg/kg. He was discharged in good general condition after 12 days.
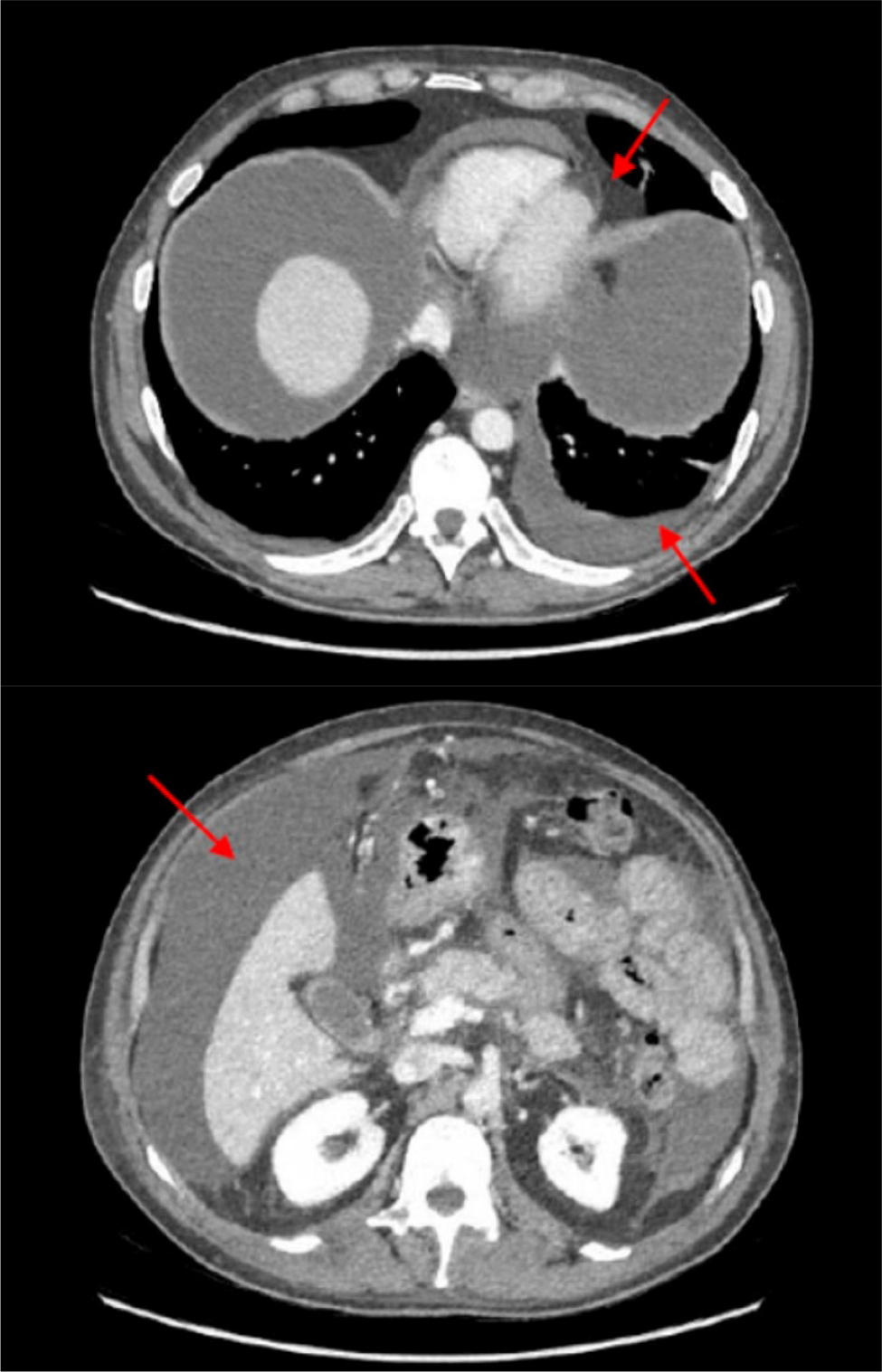
Two weeks later, in January 2023, the patient was hospitalised again due to swelling of his legs, a weight gain of 9 kg and fatigue. A new CT scan confirmed the absence of disease relapse but showed massive ascites, pleural and pericardial effusion (Fig. 1). A diagnostic paracentesis was performed, draining 500 ml of chylous fluid. Analysis of the fluid indicated a notable presence of lymphocytes suggestive of acute inflammation, with no malignant cells detected. Laboratory tests were remarkable for a biochemical thyroiditis (TSH 0.22 mcU/ml, FT4 1.21 ng/dl, FT3 1.88 pg/ml), hypoalbuminemia (2.8 g/dl) and elevated cytolysis, and necroinflammatory and cholestatic liver test (AST/ALT 144/273 U/l, ALP/γGT 209/205 U/l, total/direct bilirubin 2.4/0.7 mg/dl).
The patient restarted steroid treatment (1 mg/kg methylprednisolone), a fat and triglycerides-free diet and received albumin along with IV furosemide, with minimal effect. Consequently, high-dose IV immunoglobulin at the dose of 400 mg/kg per day for five days was added. As a result, the patient’s condition gradually improved, demonstrated by reduced peripheral oedema and progressive weight loss. Subsequently, the patient was discharged in March 2023.
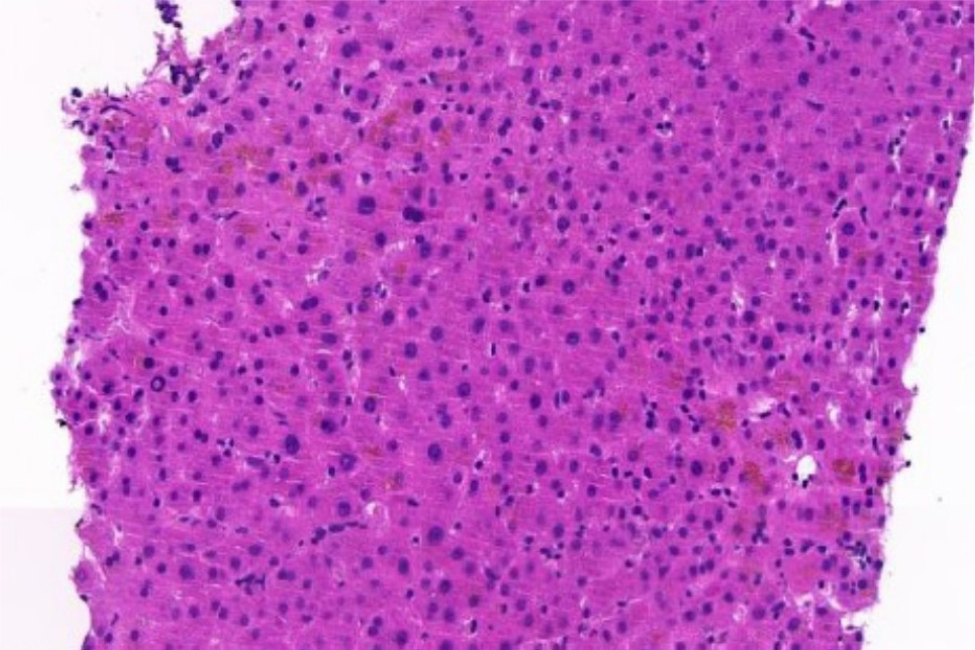
However, during the steroid tapering a new rise in transaminase and cholestasis indices (AST/ALT 193/290 U/l, γGT 1,071 U/l, ALP 348 U/l, total/direct bilirubin 2.3/0.8 mg/dl) occurred, and the patient presented again with ascites. Due to cytomegalovirus (CMV) activation (28,600 DNA copies/ml) oral valgancyclovir was started, leading to a quick decrease in viral load. Abdominal magnetic resonance imaging (MRI) and total body positron emission tomography (PET) were negative for melanoma recurrence. A transjugular liver biopsy showed scleroatrophy of the bile ducts as per previous immune-mediated hepatitis, with no signs of CMV (Fig. 2). Therefore, treatment with methylprednisolone 1 mg/kg was restored, introducing mycophenolate mofetil 500 mg bid, ursodeoxycholic acid 300 mg tid, trimethoprim-sulfamethoxazole prophylaxis and canrenone 100 mg bid. Failing to achieve a clinical response, the patient underwent a third hospitalisation in April 2023. Edoxaban was replaced with low molecular weight enoxaparin, and a paracentesis extracted 2,000 millilitres of chylous fluid. Once more, the analysis of the fluid revealed the presence of leukocytes, triglycerides and chylomicrons, without any indication of malignant circulating cells.
A new total body CT scan was performed confirming no signs of tumour recurrence, and multi-site effusions. Thick-walled lung cavitary lesions were found bilaterally and bronchoalveolar lavage was performed: cytologic analysis was negative for malignancies, culture and polymerase chain reaction (PCR) were negative for mycobacteria. The galactomannan index (0.7) was uncertain while Pseudomonas aeruginosa was isolated in cultures. As a therapeutic approach, ceftazidime 2 g qd and amphotericin B 3 mg/kg were started.
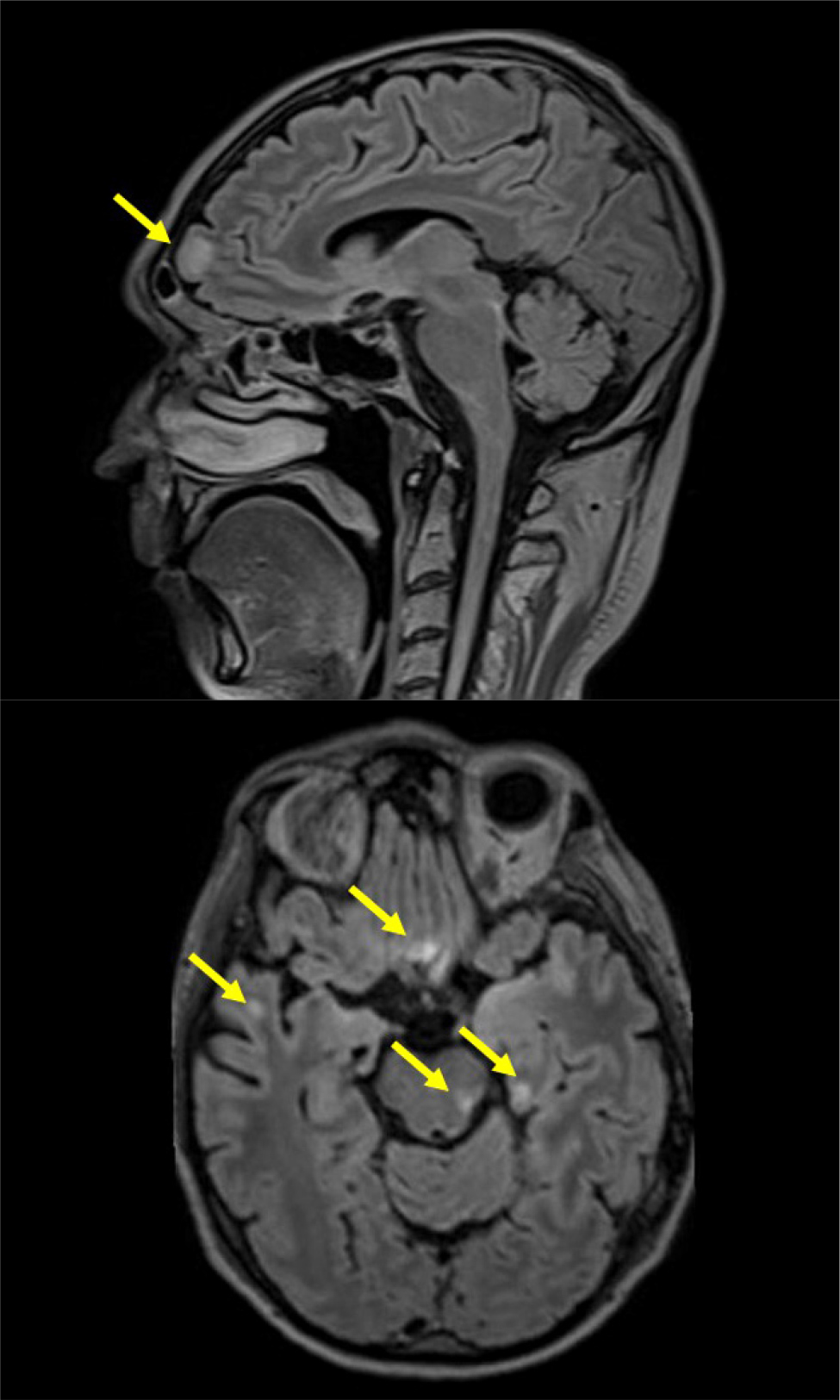
During hospitalisation the patient developed diplopia, right hypoacusis and acute urinary retention. A comprehensive ophthalmologic assessment identified impaired function of the left superior oblique muscle. Audiometry results indicated neurosensory impairment in the right ear. Although the brain CT scan upon admission appeared normal, cerebrospinal fluid analysis revealed a high count of white blood cells (77/mmc) and elevated protein concentration (153 mg/dl), along with the isolation of Varicella zoster virus. Consequently, acyclovir 750 mg tid was added. Brain and spinal cord MRI revealed T2-FLAIR hyperintensity of several cortical areas bilaterally, optic chiasm and left optic nerve, consistent with melanoma recurrence (Fig. 3). Therefore, dabrafenib and trametinib target therapy was started. On day 21 the patient suddenly developed severe hypotension and anaemia due to a spontaneous chest wall haematoma. An angiography revealed no active bleeding source. Circulatory support was initiated through blood transfusions, volume expansion and an adrenaline infusion. Haemodynamics and haematocrit progressively stabilised, while his neurological state worsened with disinhibition and cognitive impairment. Considering the progressive deterioration in his general condition and poor prognosis related to melanoma recurrence, oncological therapy was suspended, and the patient was moved to the Palliative Care Unit, where he died in May 2023.
DISCUSSION
Immune-related adverse events with ICPIs are frequent[2]. However, rare cases such as polyserositis with oedema – partly responsive to steroids – in combination with other adverse events such as hepatitis and thyroiditis, along with tumour progression after discontinuation of ICPIs, have been less frequently documented in case series[3]. The clinical manifestations of our patient are peculiar and suggest a severe endothelial dysfunction, both vascular and lymphatic. Based on the adverse drug reactions (ADR) probability scale[4], which yielded a total score of 5 indicating a probable ADR, we reasonably associated these signs and symptoms with the administration of nivolumab treatment.
Some case reports described polyserositis consistent with secondary capillary leak syndrome[5,6], but in our case typical signs such as hypotension and haemoconcentration were not present.
Additionally, the identification of isolated brain metastases occurred five months after the initial admission. Notably, only a few instances of chylous ascites associated with chylothorax have been previously reported[7], with another patient developing solely chylothorax[8]. In all these cases, high-dose IV steroids were used with a wide range of clinical responses; different immunomodulating therapy trials were given, obtaining complete remission in one case.
CONCLUSION
A considerable percentage of oncological patients are eligible for ICPIs[9], therefore adverse events – including extremely rare toxicities – are expected to increase. Parameters to identify patients at risk of severe toxicities and prospective data about different immunomodulating therapies efficacy are still lacking. Further studies on the likelihood of response and the risk of toxicity are needed to evaluate the risk/benefit ratio of treatment with ICPIs.