ABSTRACT
Primary pulmonary T-cell lymphoma (PPTL) is a rare disease. Diagnosing PPTL is challenging due to non-specific clinical symptoms and imaging. A 32-year-old female presented with persistent fever, cough, and dyspnoea. The symptoms were initially treated as asthma and community-acquired pneumonia without improvement. Chest computed tomography (CT) revealed bilateral consolidations with a CT angiogram sign, and flexible bronchoscopy showed infiltrative lesions causing bronchial stenosis. Histopathological examination of the tissue biopsy identified T-cell lymphoma through immunohistochemical staining positive for CD3. This case highlights the importance of considering differential diagnoses such as PPTL in patients with atypical presentations of asthma or non-resolving pneumonia. This case also demonstrates the diagnostic utility of flexible bronchoscopy in identifying airway obstruction due to malignant cells, which can mimic asthma.
LEARNING POINTS
- Primary pulmonary T-cell lymphoma can manifest as atypical asthma and non-resolving pneumonia, making early diagnosis challenging.
- Malignant aetiologies, including primary pulmonary T-cell lymphoma, should be considered in cases of bilateral consolidations that do not respond to antibiotics and present CT angiogram signs.
- Histopathology remains the gold standard in primary pulmonary T-cell lymphoma diagnosis, wherein flexible bronchoscopy should be employed as a minimally invasive first-line approach for tissue biopsy.
KEYWORDS
Primary pulmonary T-cell lymphoma, asthma, CT angiogram sign, non-resolving pneumonia
INTRODUCTION
Primary pulmonary lymphoma is characterized by the proliferation of lymphoid cells within the lung without evidence of lymphoma elsewhere at the time of diagnosis and during a minimum follow-up period of three months. Primary pulmonary lymphoma is a rare disease, accounting for less than 1% of non-Hodgkin’s lymphoma cases and less than 0.5% of primary lung cancers[1]. Primary pulmonary lymphoma of B-cell origin is the most common subtype, accounting for 80% of cases, while cases of primary pulmonary lymphoma of T-cell origin are less common[1]. Lymphoma of T-cell origin tends to have a worse prognosis, progress more rapidly, and have a higher mortality rate. Diagnosing pulmonary T-cell lymphoma (PPTL) is challenging due to non-specific clinical symptoms and imaging. We report a case of PPTL that was initially misdiagnosed as asthma and community-acquired pneumonia.
CLINICAL CASE
A 32-year-old female was admitted to Cho Ray Hospital, Ho Chi Minh City, Vietnam due to a persistent fever lasting for the past month. One year before hospitalization, the patient experienced an episode of dyspnoea and wheezing and was diagnosed with asthma. The treatment involved inhaled corticosteroids/long-acting beta-agonists (ICS/LABA) and oral corticosteroids without performance spirometry. The patient received asthma treatment despite occasionally reporting exertional dyspnoea. The patient’s medical history revealed no allergies, malignancies, anaemia, lymphadenopathy, or smoking. The patient reported no family history of such diseases.
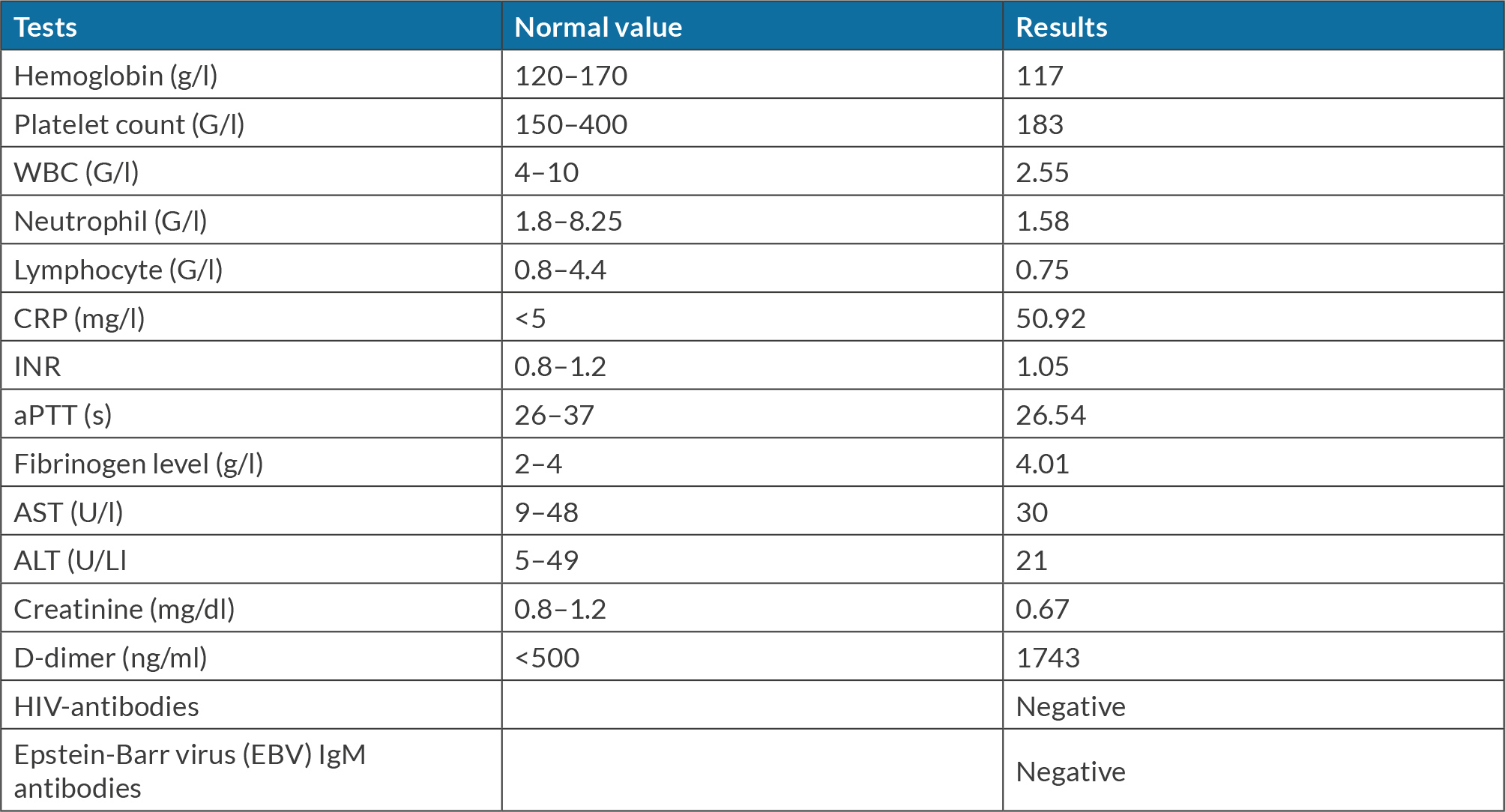
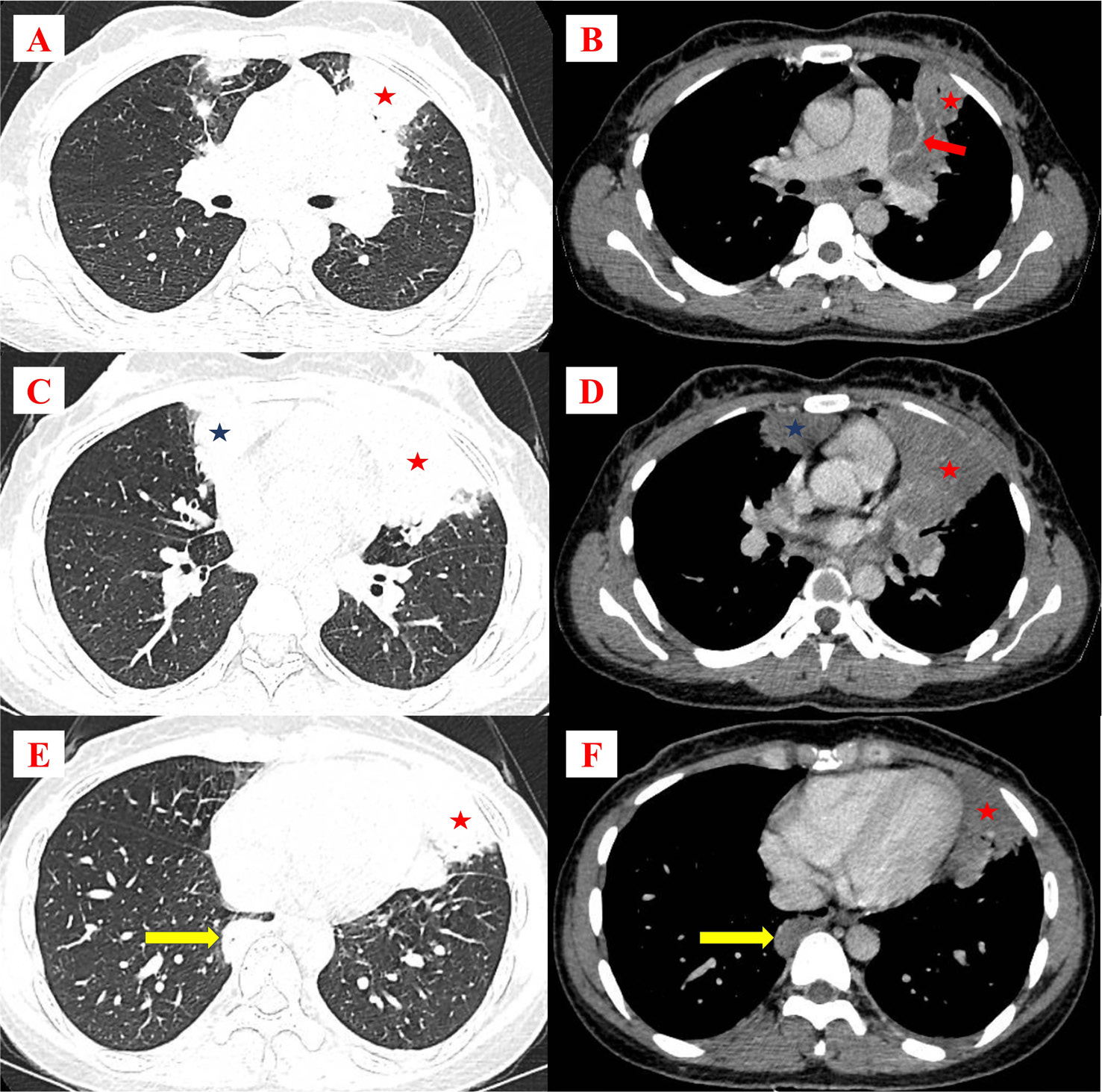
At Cho Ray Hospital, the patient presented with a continuous fever of 39°C. Despite a five-day outpatient regimen of oral antibiotics, including amoxicillin/clavulanic acid and levofloxacin, the fever was not improved before hospitalization. The patient also reported non-productive cough, pleuritic chest pain, and significant weight loss. Clinical examination showed normal vital signs and oxygen saturation (SpO2) of 96% in room air. No lymphadenopathy was detected in the neck, armpits, or groin. Upon lung auscultation, wheezing was heard during exhalation in the upper regions bilaterally. The results of laboratory tests indicated leukopenia and elevated C-reactive protein levels (Table 1). Chest X-ray showed a heterogeneous opacity occupying the mid and lower zones of the left lung, obliterating the left cardiac border (Fig. 1). Chest computed tomography (CT) showed two separate consolidations in the right and left upper lobes (S3) characterized by heterogeneous contrast enhancement and a solid lesion adjacent to the right pleura. No pleural effusion or mediastinal lymphadenopathy was identified (Fig. 2).
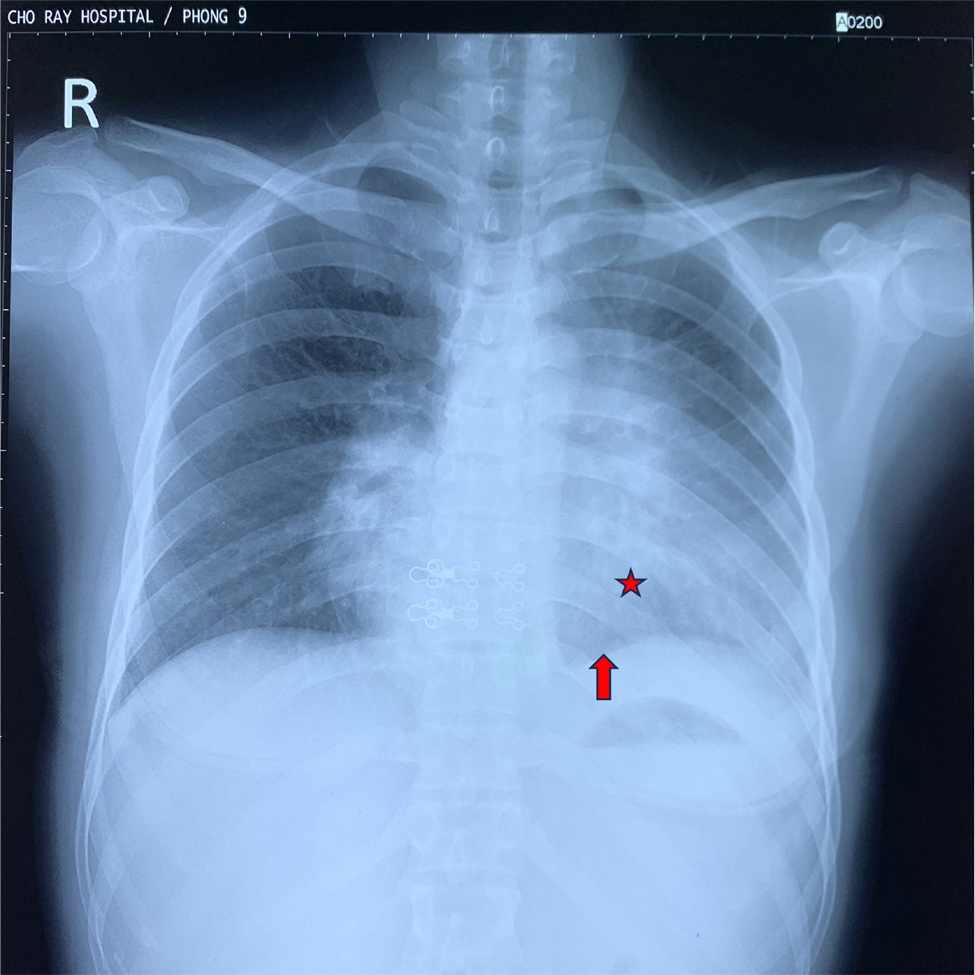
Figure 1. Chest X-ray of the patient. The image reveals a heterogeneous opacity occupying the mid and lower zones of the left lung, causing the left cardiac border to be obscured (red star). The left diaphragm was approximately equal to the right hemidiaphragm, indicating potential lung collapse or subpulmonary effusion (red arrows).
Figure 2. Chest CT of the patient. Figures A and B show a consolidation in the left upper lobe (red stars) with CT angiogram sign (red arrows). Figures C and D display two separate consolidations in the left (red stars) and right upper lobe (blue stars) with heterogeneous contrast enhancement. Figures E and F show a solid lesion adjacent to the right pleura (yellow arrows).
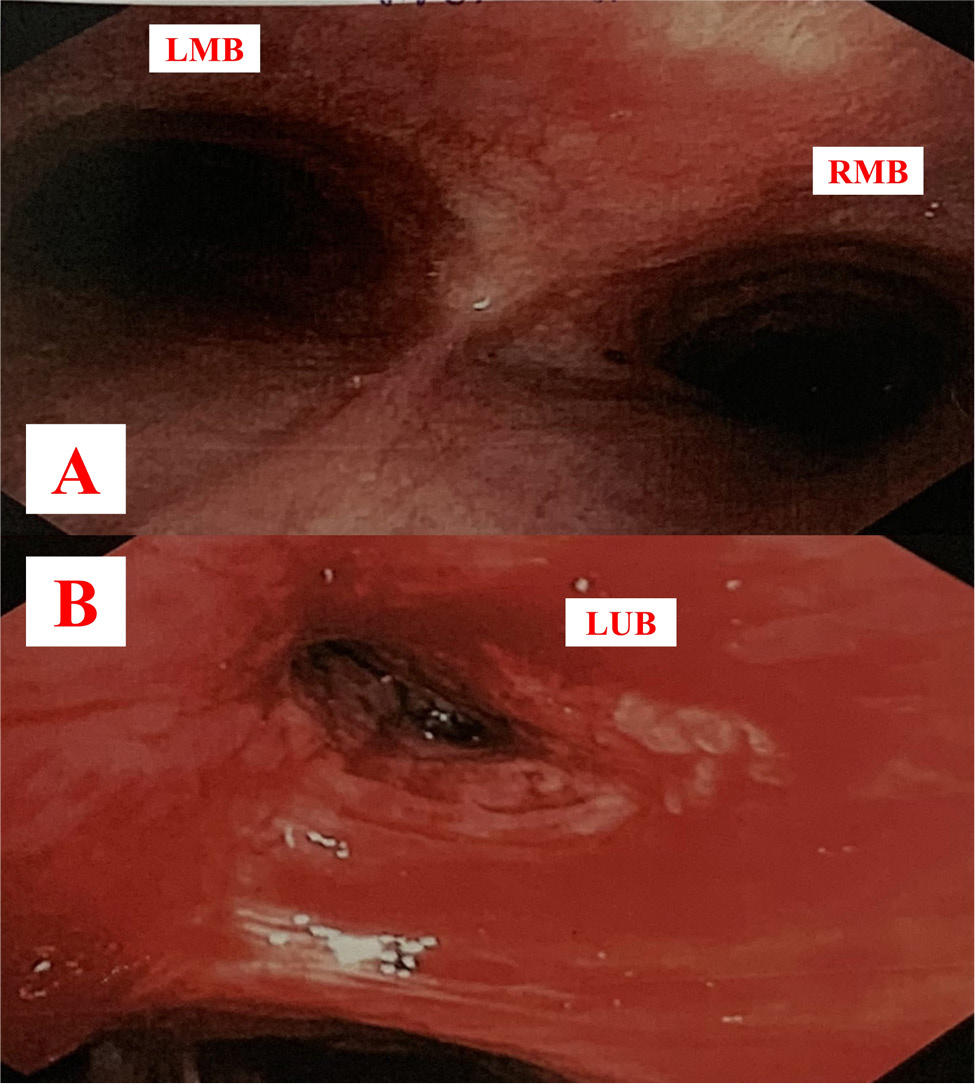
The patient was initially treated with intravenous antibiotics, including cefoperazone/sulbactam and levofloxacin, for three days. Subsequently, the treatment was switched to meropenem and vancomycin due to the patient’s ongoing fever and persistent dry cough. On the fifth day, a flexible bronchoscopy was performed. The bronchoscopy revealed marked infiltrated lesions and constriction of the left upper lobe bronchus (Fig. 3). A bronchial mucosal biopsy was performed, and bronchoalveolar lavage fluid was sent for cytological and microbiological analysis. The result of acid-fast bacilli (AFB) smear, Xpert, fungal culture, bacterial culture, and Mycobacteria tuberculosis polymerase chain reaction (PCR) were all negative. Figure 4 illustrates histopathological findings, with immunohistochemical staining of tumour cells revealing CD3 (+), CD20 (-), pan-cytokeratin (-), TTF-1 (-), and chromogranin-A (-). These results suggest a malignant origin of T-lymphocyte cells. CT scans of the pelvic and abdominal regions showed normal findings. Bone marrow biopsy was not performed. The patient declined further treatment, requested discharge, and passed away one month later.
Figure 3. Flexible bronchoscopy images. Figure A shows normal bronchial mucosa in two main bronchi. Figure B shows an infiltrated lesion causing marked constriction of the left upper lobe bronchus. LMB: left main bronchus, RMB: right main bronchus, LUB: left upper bronchus.
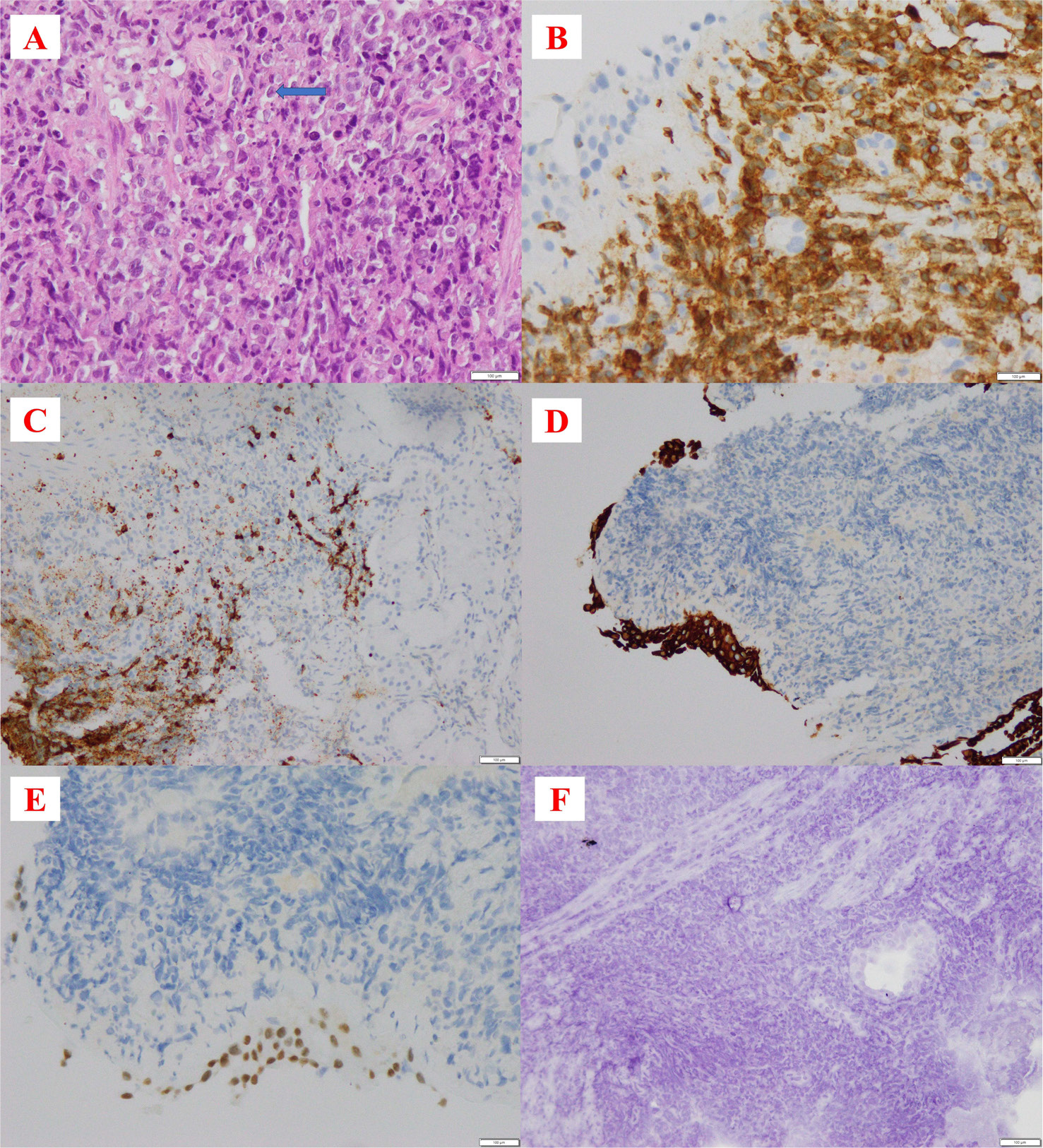
Figure 4. Histopathology of bronchial tissue. Figure A (HE stain 40X): the tissue includes lymphoid cells with large nuclei, sparse cytoplasm, and a scattered arrangement. Some cells have prominent nuclei (blue arrow). Figure B (CD3 stain 40X): the membranes of tumour cells were stained diffusely. Figure C (CD20 20X), Figure D (pan-cytokeratin stain 20X), Figure E (TTF-1 40X), Figure F (chromogranin-A 20X): tumour cells do not stain.
DISCUSSION
PPTL is an extremely rare malignancy. Our patient is the first PPTL case initially misdiagnosed as asthma. Over-diagnosing asthma is not uncommon in clinical practice. Furthermore, malignancy is not a typical differential diagnosis for asthma, especially in young, non-smoking patients. The asthma diagnosis should be reconsidered when patients are admitted to hospital with symptoms unresponsive to ICS/LABA, lack spirometry test results, have no recorded allergies, and have localized wheezing on lung auscultation. Flexible bronchoscopy confirmed the source of wheezing in narrowing of the left upper bronchus caused by cancer cells. Sirmali et al. reported a case of high-grade B-cell lymphoma initially misdiagnosed as asthma, where bronchoscopy revealed narrowing of the right intermediate lobe bronchus[2].
Published case series demonstrate numerous instances of PPTL initially managed as pneumonia. There are two reasons for the misdiagnosis. Firstly, the clinical symptoms of most PPTL cases, including fever, non-productive cough, dyspnoea, and pleuritic chest pain, closely resemble those of pneumonia[3-5]. Secondly, imaging of PPTL often exhibit “pneumonia-like features”, characterized by consolidation with air bronchograms and diffuse distribution[3,4]. Although there is no international consensus, early bronchoscopy within 3-4 days has been proposed in cases of non-resolving pneumonia[6].
PPTL presents non-specific radiological manifestations. Reported PPTL lesions on imaging include multiple lung nodules, pleural effusion, consolidation with air bronchogram, mass with central necrosis[5], ground-glass opacity, or subpleural consolidation[3,7]. Mediastinal lymphadenopathy is infrequent, accounting for 9% of cases[4]. The most common PPTL presentation is multiple nodules distributed in both lungs, accounting for 53.3% of cases[3]. Takahara et al. suggests that the non-specific radiologic characteristics observed in PPTL cases are due to atypical lymphoid cells that can infiltrate the lungs through three different pathways: infiltration into lymphatic vessels, direct invasion, or hematogenous metastasis[8]. CT angiogram sign has been described as a potential clue for mucosa-associated lymphoid tissue pulmonary lymphoma originating from B-cell lymphocytes[9]. However, there have been no cases reported with CT angiogram signs in PPTL.
The diagnostic yield of pulmonary lymphoma through flexible bronchoscopy is generally low[10]. Biopsies of bronchial mucosae are rare. Transbronchial lung biopsies are more frequent, but have limited diagnostic capability due to the small size of tissue samples obtained[5]. One report revealed a diagnostic failure rate of 9/11 cases with transbronchial lung biopsies[4], while another study reported a diagnostic success rate of 3/15 cases with this method[3]. In most cases, PPTL is confirmed through open lung biopsy or video-assisted thoracoscopic surgery[3]. However, flexible bronchoscopy maintains value in obtaining bronchoalveolar lavage samples that can be used to identify causative pathogens and exclude differential diagnoses. Although its diagnostic value for PPTL is relatively low, flexible bronchoscopy represents a less invasive approach that helps reduce the risk of complications.
CONCLUSION
Our case highlights the challenges in diagnosing PPTL due to the rarity and non-specific clinical symptoms and imaging of this disease. PPTL should be considered in cases of community-acquired pneumonia unresponsive to initial antibiotic treatment, accompanied by chest CT findings of nodular and consolidations with CT angiogram signs. Flexible bronchoscopy should be considered, especially when malignant cell airway obstruction is suspected. PPTL carries a poor prognosis, emphasizing the need for early diagnosis to improve outcomes.