ABSTRACT
Pituitary apoplexy is an uncommon condition typically resulting from a sudden haemorrhage within a pituitary adenoma. This bleed can present clinically with a wide array of signs and symptoms. This report documents the case of a 62-year-old male who presented to the Lebanese Hospital Geitaoui University Medical Center with signs and symptoms of meningeal irritation. He was initially thought to have meningitis, and was started on antibiotics; he was then found to have pituitary adenoma apoplexy that was complicated by syndrome of inappropriate antidiuretic hormone release (SIADH). The patient was successfully treated with antibiotics, and fluid restriction and hypertonic saline after ruling out other more common causes for his hyponatraemia, before undergoing a transsphenoidal resection of the pituitary adenoma. A three-month follow-up evaluation of the patient demonstrated the absence of hormonal imbalances and the absence of residual tumours on imaging.
LEARNING POINTS
- Pituitary apoplexy has as a wide clinical presentation
- Pituitary apoplexy should be ruled out in patients with aseptic chemical meningitis with a history of pituitary adenomas
- SIADH can complicate chemical meningitis due to pituitary apoplexy
KEYWORDS
Pituitary adenoma, pituitary apoplexy, SIADH, chemical meningitis
INTRODUCTION
Pituitary apoplexy (PA) is a condition resulting from the sudden haemorrhage or infarction of the pituitary gland[1]. A haemorrhage often occurs within pituitary adenomas, as these adenomas account for 10% of all intracranial masses, particularly in patients over the age of 30[2,3]. The abrupt expansion of the pituitary mass following the sudden bleed causes acute clinical findings such as nausea, decreased visual loss and diplopia, thunderclap headaches, decreased level of consciousness, post-operative visual loss, panhypopituitarism and syndrome of inappropriate antidiuretic hormone release (SIADH) leading to endocrine emergencies[4-7]. The expulsion of necrotic pituitary cells through the sellar diaphragm aperture can lead to meningeal irritation, thereby causing chemical meningitis, clinically indistinguishable from bacterial meningitis or subarachnoid haemorrhage[8], leading to diagnostic and therapeutic delays. Meningeal irritation signs are a rare clinical presentation of PA, and existing literature predominantly comprises isolated case reports. In a retrospective study of 255 patients with pituitary tumours, only seven patients had meningeal manifestations as the initial sign of PA[9]. Furthermore, SIADH is a rarely reported manifestation resulting from PA, causing hyponatraemia. A thorough search of the PubMed database using keywords ‘pituitary apoplexy’ and ‘SIADH’ yielded just four documented case reports. We present a unique case, in which PA presented with features of chemical meningitis and posterior pituitary dysfunction causing hyponatraemia secondary to SIADH – a presentation that has not been previously documented.
CASE DESCRIPTION
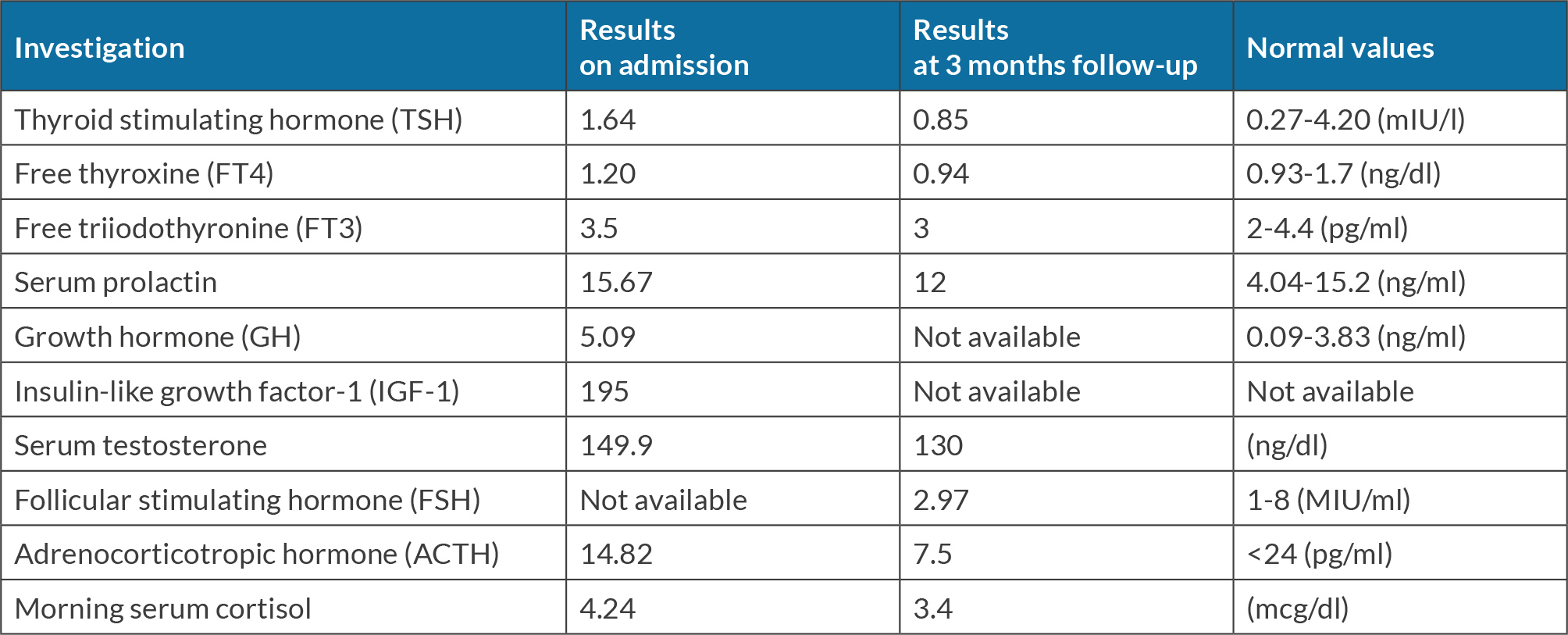
A 62-year-old male patient presented to the emergency department of the Lebanese Hospital Geitaoui University Medical Center (LHG-UMC), with an acute severe headache, impaired consciousness and decrease visual acuity. His past medical history is significant for hypertension, dyslipidaemia, diabetes mellitus and coronary artery disease status post percutaneous coronary angioplasty. Upon presentation the patient was lethargic, alert but disoriented. His temperature was 38.5°C. Clinical assessment revealed a Glasgow coma scale (GCS) score of 14 (eye opening: 4; verbal response: 4; motor response: 6). Physical examination showed limitation of neck flexion, but no neck stiffness. Brudzinski and Kernig signs were negative. An urgent cranial computed tomography (CT) scan (Fig. 1) performed without the administration of contrast medium revealed a heterogeneous sellar nodular lesion. A dedicated pituitary magnetic resonance imaging (MRI) scan was ordered the next day for better evaluation of the lesions’ signal characteristics and mass effect on adjacent structures. Initial laboratory studies revealed a haemoglobin level of 14.1 g/dl (reference range 14–18 g/dl), leukocytosis at 12,840/mm3 (reference range 4,800–10,800/mm3), and an elevated C-reactive protein of 73 mg/l (reference range <6 mg/l). His sodium level was low at 122 mmol/l (reference range 136–145 mmol/l). Liver and kidney function tests were normal. A lumbar puncture showed cerebrospinal fluid (CSF) with a white blood cell count of 870/mm3 (91% polymorphs, 4% lymphocytes), total protein of 168.5 mg/dl and glucose of 79 mg/dl. The patient was started on intravenous antibiotics with ceftriaxone and vancomycin due to suspicion of meningitis and he was also considered to have an incidental pituitary adenoma. Dexamethasone (24 mg/day) was added to control the pituitary adenoma mass effect on parasellar structures. A hormone profile (Table 1) showed normal thyroid function and morning serum cortisol level. Serum prolactin and serum growth hormone were also within normal limits, confirming a non-functioning pituitary adenoma.
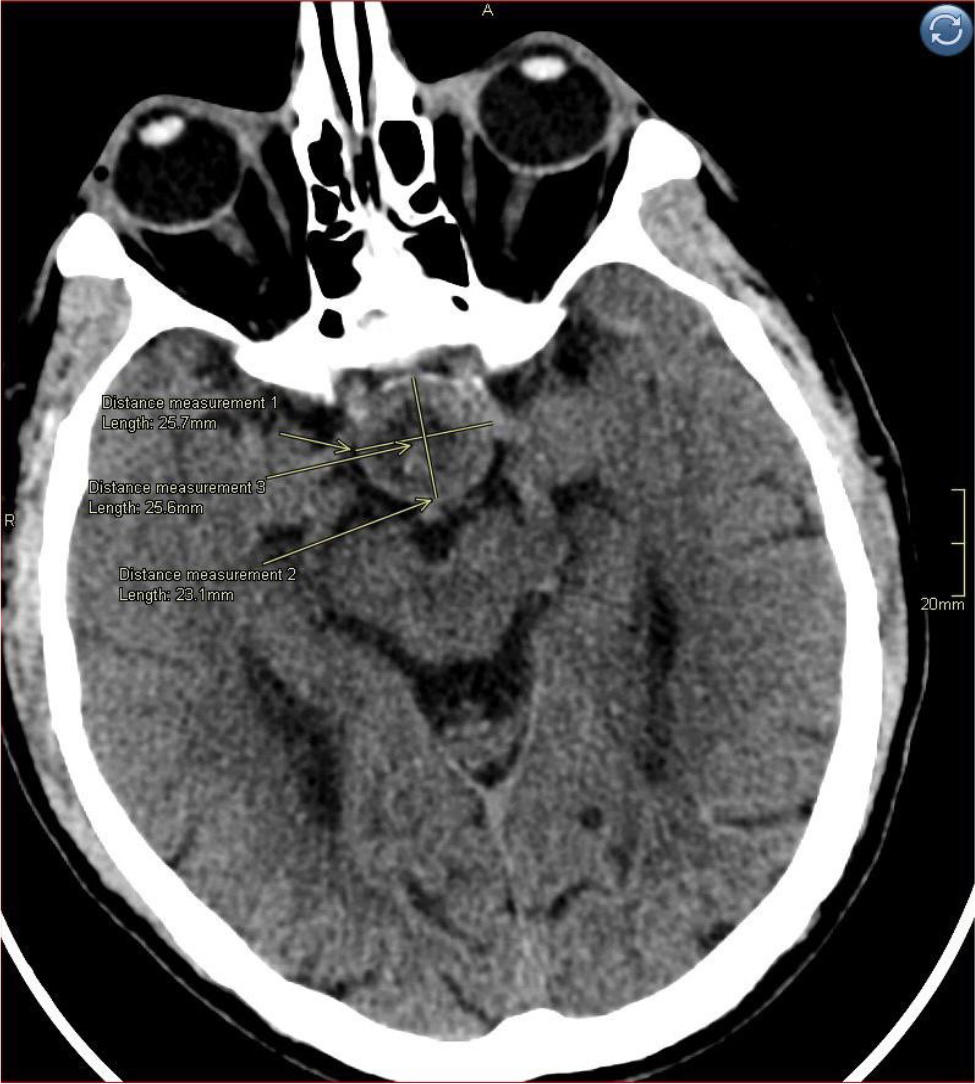
Figure 1. Non-contrast CT scan of the brain on admission demonstrating a heterogenous sellar nodular lesion measuring 25 × 24 mm.
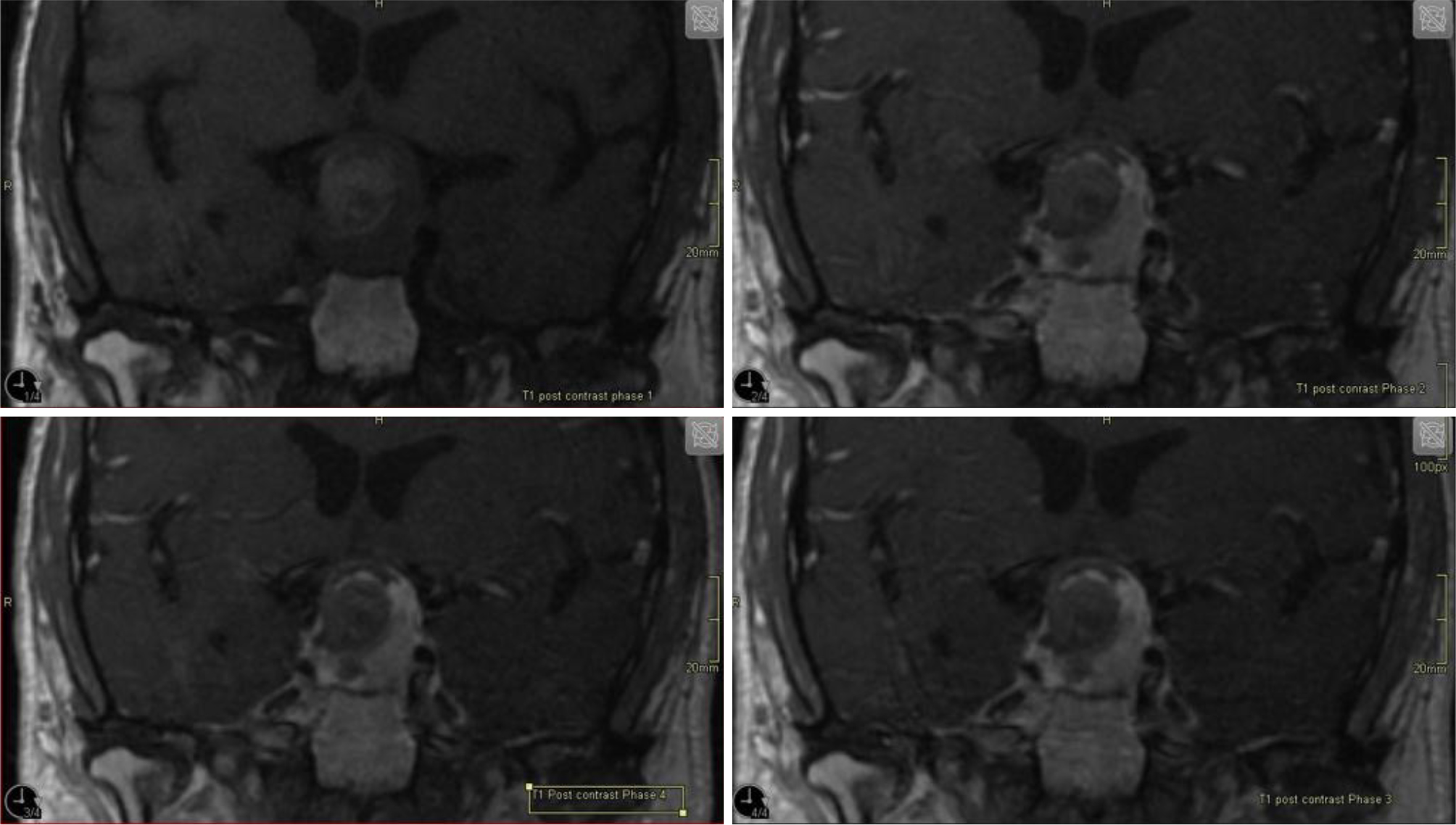
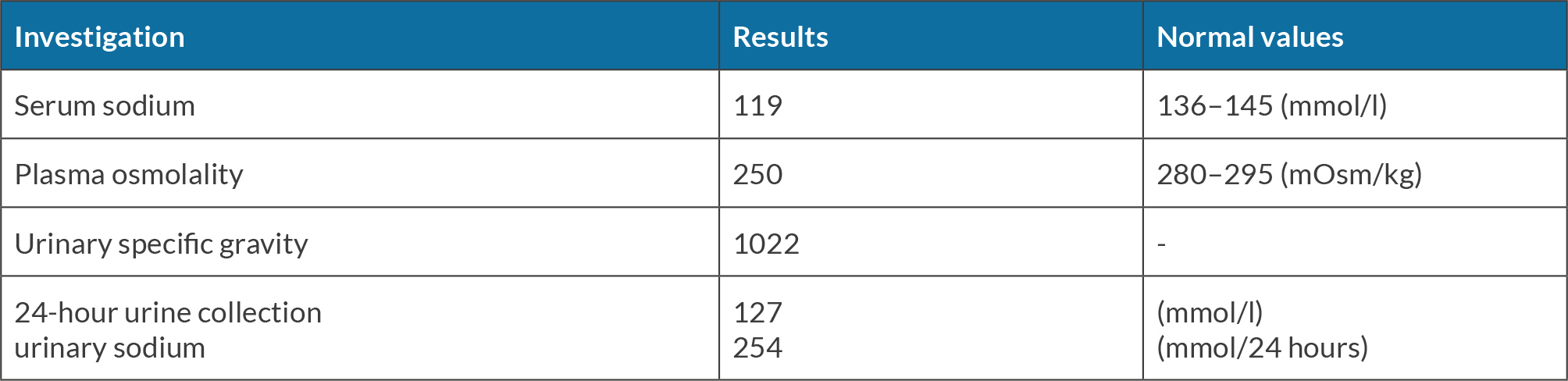
A pituitary MRI (Fig. 2) revealed a solid and suprasellar mass exhibiting mixed signal intensity with a peripheral fibrous component, a central component of haemorrhage, in keeping with pituitary haemorrhagic apoplexy of the pituitary adenoma (Fig. 2). A perimetry test for the assessment of the visual field revealed bitemporal hemianopia. Laboratory investigation at day 3 (Table 2) revealed a sodium level at 119 mmol/l (reference range 136–145 mmol/l). Serum and urine osmolarity confirmed the diagnosis of SIADH. The hyponatraemia was corrected by fluid restriction and infusion of hypertonic saline (3%) over the following few days. Blood culture and CSF cultures came back negative after 8 days of incubation.
Figure 2. Coronal T1-weighted MRI with IV gadolinium centred over the pituitary gland (dynamic) demonstrating haemorrhagic area within the pituitary adenoma.
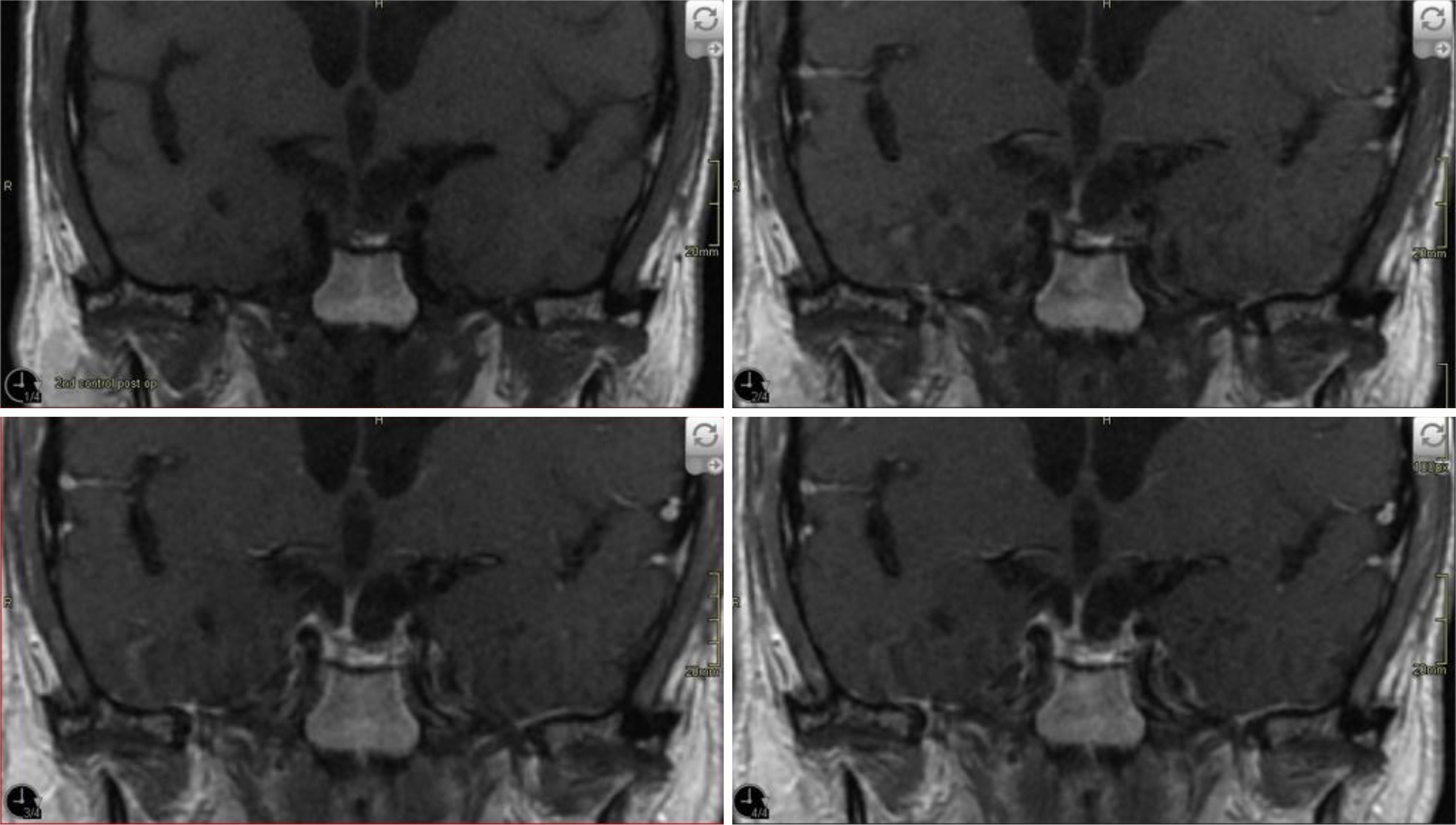
The patient underwent transsphenoidal resection of the adenoma after correction of his hyponatraemia and completion of the antibiotic course. He reported improved visual acuity in the immediate post-operative period, though bitemporal field cuts persisted. Histopathologic evaluation confirmed the tumour to be a pituitary adenoma. (Fig. 3). Three months after the operation, the patient had no significant decrease in pituitary function and required no hormone replacement (Table 1). A repeated visual field test was within normal limits, and a follow-up brain MRI (Fig. 4) at 3 months revealed no evidence of residual or recurrent tissues at surgical sites.
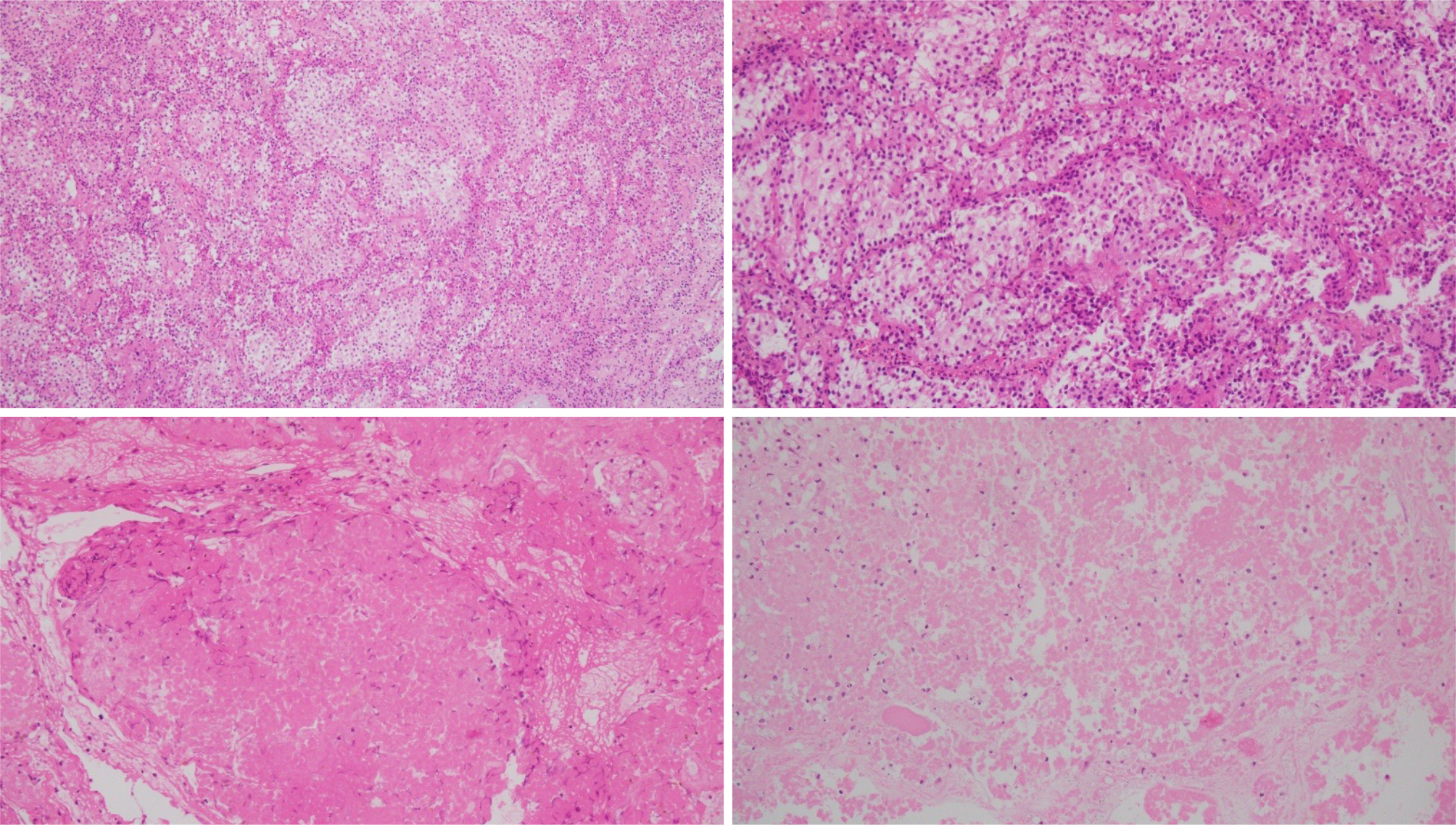
Figure 3. Histologic appearance of the pituitary tumour with necrosis and inflammation, with haematoxylin-eosin staining.
Figure 4. Follow-up MRI with gadolinium at 3 months demonstrating no evidence of residual or recurrent tissue.
DISCUSSION
PA is a rare condition, with an estimated prevalence of 6.2 cases per 100,000 inhabitants reported in a large epidemiological study in the UK[10]. In a retrospective study involving 1,067 patients with pituitary adenoma, symptomatic PA was identified in 43 patients (4%)[11]. The incidence rate varies substantially, ranging from as low as 1.6% as reported in one of the largest studies of pituitary adenoma over a 35-year period[12], to as high as 16.6% reported by other researchers[13]. PA most frequently presents with sudden onset of a severe headache in over 80% of patients and visual defects in more than half of the cases[14]. In this case, we describe a male patient in his sixties, originally misdiagnosed with meningitis, who presented with signs of meningeal irritation: fever, headache and CSF pleocytosis, which eventually proved to be sterile. He was subsequently diagnosed with pituitary adenoma apoplexy following an MRI scan. The clinical manifestation of meningitis can be attributed to the leakage of the necrotic and haemorrhagic material into the Suprasellar subarachnoid space. Although rare, chemical meningitis can be the major clinical presentation of PA[15]. In a retrospective study that included 37 patients with PA, CT scans successfully identified pituitary haemorrhage in only 46% of the cases[16]. Similar to our case, a CT scan revealed the presence of a pituitary tumour; however, it failed to identify any haemorrhagic components within it. MRI is considered to be superior to a CT scan in the diagnosis of PA with a reported sensitivity ranging from 88% to 90%[17]. However, a CT scan seems to be the preferred initial examination in emergency cases characterised by a sudden onset of headache. This choice assists in excluding more common differential diagnoses, such as subarachnoid haemorrhage. Apoplexy occurs more frequently in macroadenomas and non-functioning pituitary adenoma. This could be related in part to macroadenomas being less vascularised vis-à-vis the pituitary gland, and to the rapid growth in macroadenomas leading to ischaemic necrosis and subsequent haemorrhage. Nevertheless, PA has been reported in microadenomas as well[18,19]. The initial serum sodium level was at 125 mmol/l, and the patient’s hyponatraemia was initially presumed to be attributed to corticotropin deficiency causing adrenal insufficiency, so empirical steroids were initiated. However, endocrinological studies came back negative the next day (Table 1), and the serum sodium level continued to decrease, dropping to a nadir level of 119 mmol/l. When volume status was evaluated, the patient was found to be euvolemic; he did not have crackles on lung auscultation nor lower limb oedema, and urine and serum examination (Table 2) were consistent with SIADH at day 3. Posterior pituitary dysfunction is a rare manifestation of PA. It is speculated that compression of the internal carotid within the cavernous sinus could hinder blood supply to the supraoptic nuclei and/or infundibular process, leading to ischaemic insults to the areas of antidiuretic hormone synthesis and release[20]. Our patient had decreased visual acuity, and the MRI report revealed compression and superior displacement of the optic chiasm, along with lateral protrusion of the tumour. Moreover, the patient responded well to SIADH treatment. PA, albeit rare, should always be considered in a patient presenting with signs of meningeal irritation. While hyponatraemia can be secondary to hypothyroidism or cortisol deficiency, SIADH should also be considered as an aetiology of hyponatraemia in PA.
CONCLUSION
This is the first documented case of PA complicated by chemical meningitis and SIADH. Clinicians should stay vigilant when patients with known pituitary adenomas present with meningeal irritation signs and signs of hyponatraemia.