ABSTRACT
Coronary cameral fistula (CCF) is defined as an abnormal connection between the coronary artery and any cardiac chamber. It usually appears due to abnormal embryogenesis and represents less than 1% of the population. Most CCF cases are asymptomatic, however large CCFs may cause symptoms and complications. We present a case of a young female with symptomatic CCF suspected on echocardiography and confirmed by computed tomography coronary angiography. She was successfully treated surgically with total improvement of symptoms.
LEARNING POINTS
- Coronary cameral fistula (CCF) is an abnormal communication between the coronary arteries and cardiac chambers. Most fistulas are asymptomatic, however, large CCFs can cause symptoms.
- CCF can cause angina pectoris due to the coronary steal phenomenon, and may require surgical intervention.
- Coronary computed tomography angiography is a highly sensitive method for detecting coronary artery fistulas noninvasively.
KEYWORDS
Angina pectoris, Coronary cameral fistula, coronary steal phenomenon, Coronary artery bypass graft
INTRODUCTION
Abnormal embryogenesis is considered the reason for most cases of coronary cameral fistula (CCF). However, other iatrogenic causes may lead to this abnormal communication, such as trauma (stab injury), invasive procedures (coronary angiography, pacemaker implantation), or cardiac surgery (septal myomectomy). In total, 0.2 to 0.4% of congenital anomalies of the heart result in CCF. Fistulas can be described by their site of origin and site of termination. In our case, a young female had recurrent anginal pain as a result of the coronary steal phenomenon due to large abnormal communication between the left circumflex artery and right atrium. Surgical treatment with closure of the fistula site and coronary artery bypass graft were successful in alleviating symptoms.
CASE DESCRIPTION
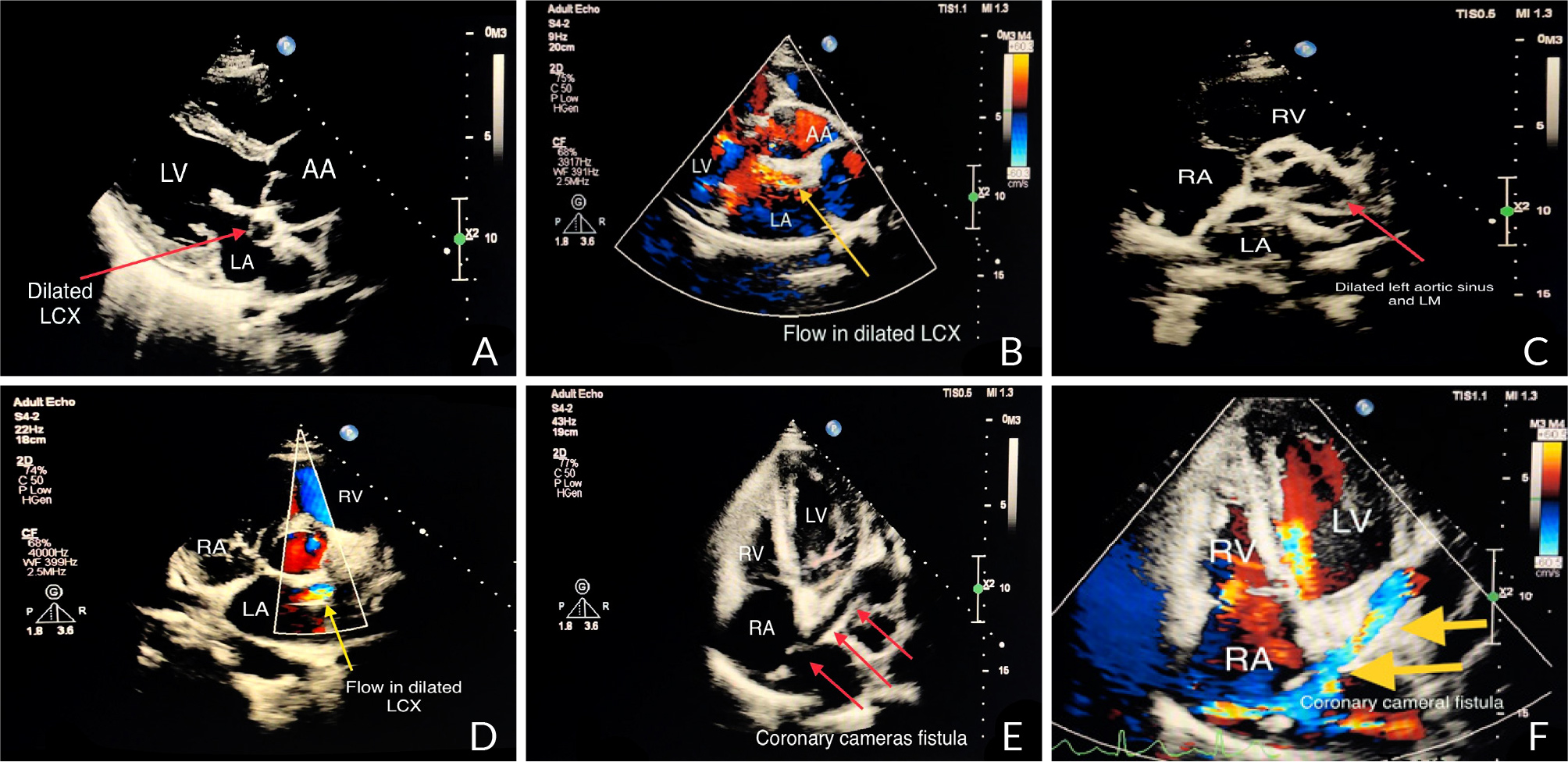
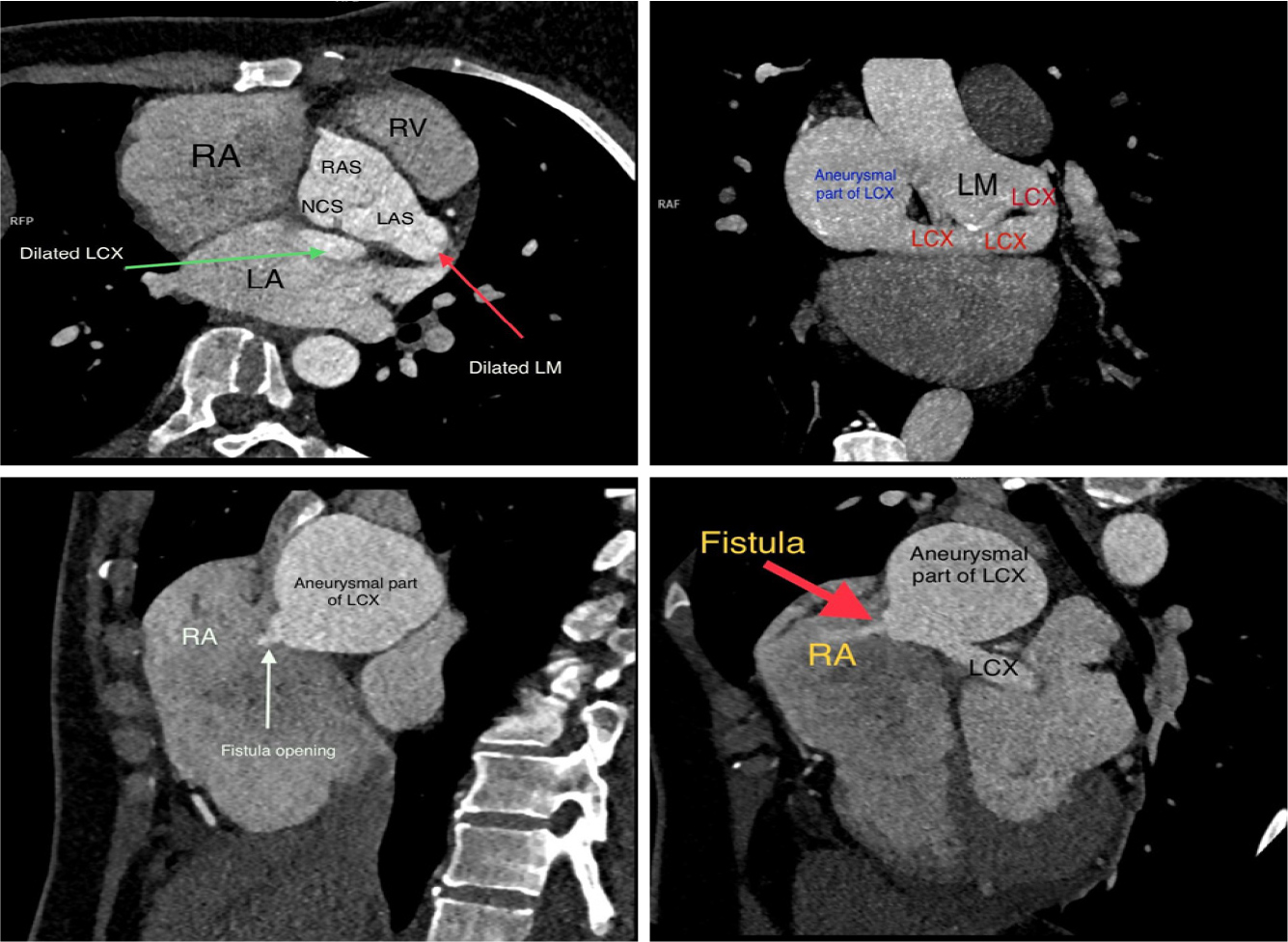
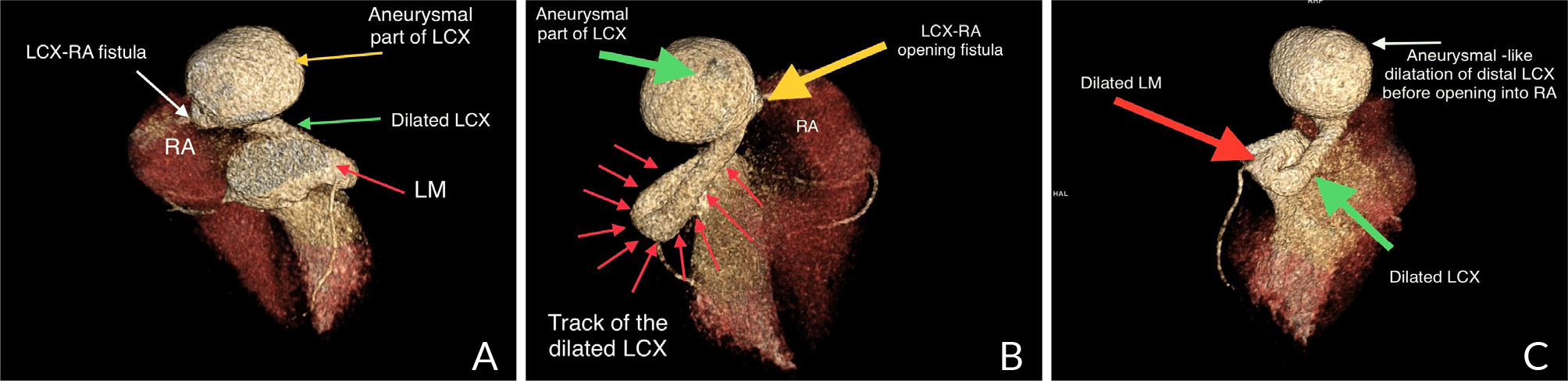
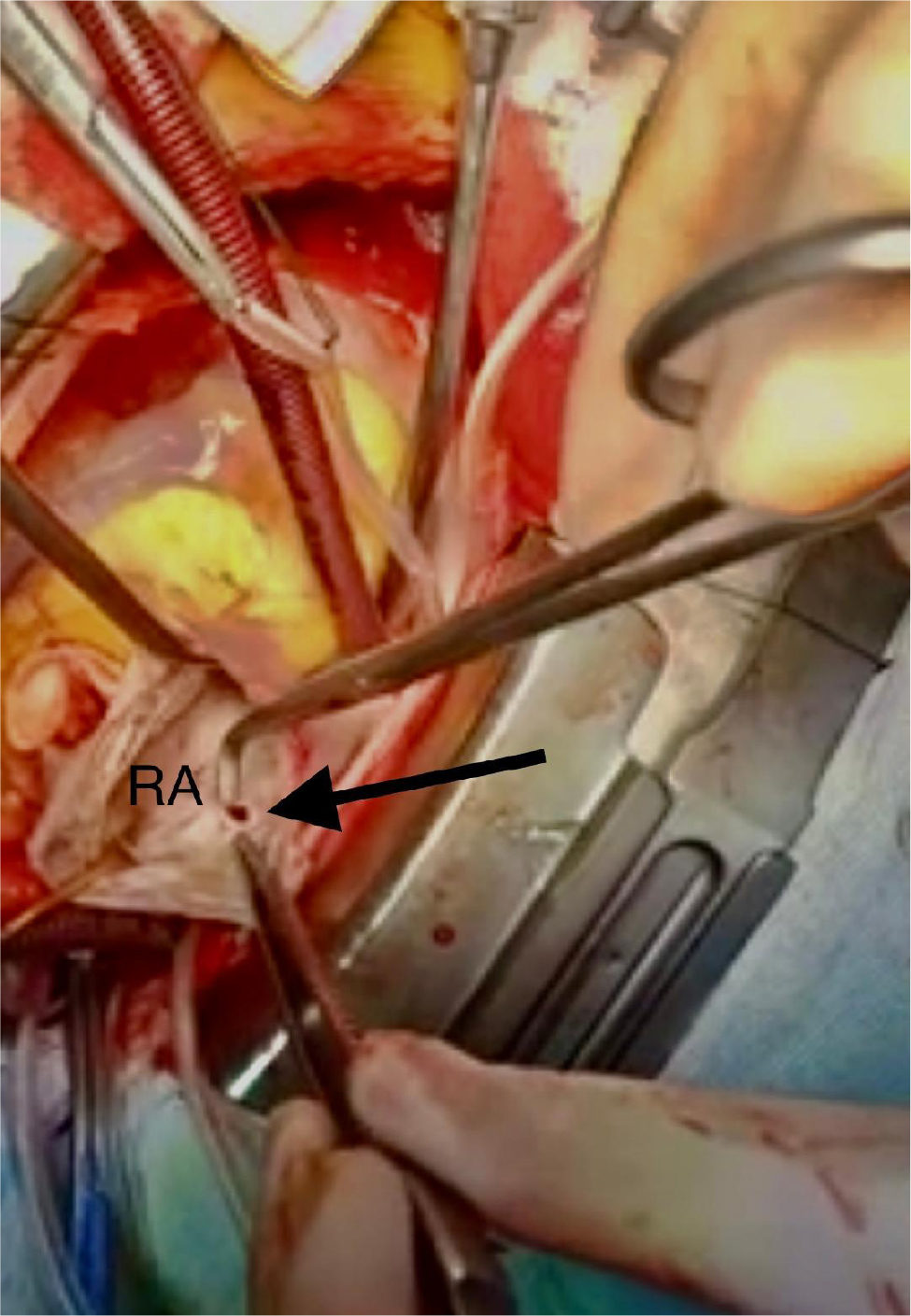
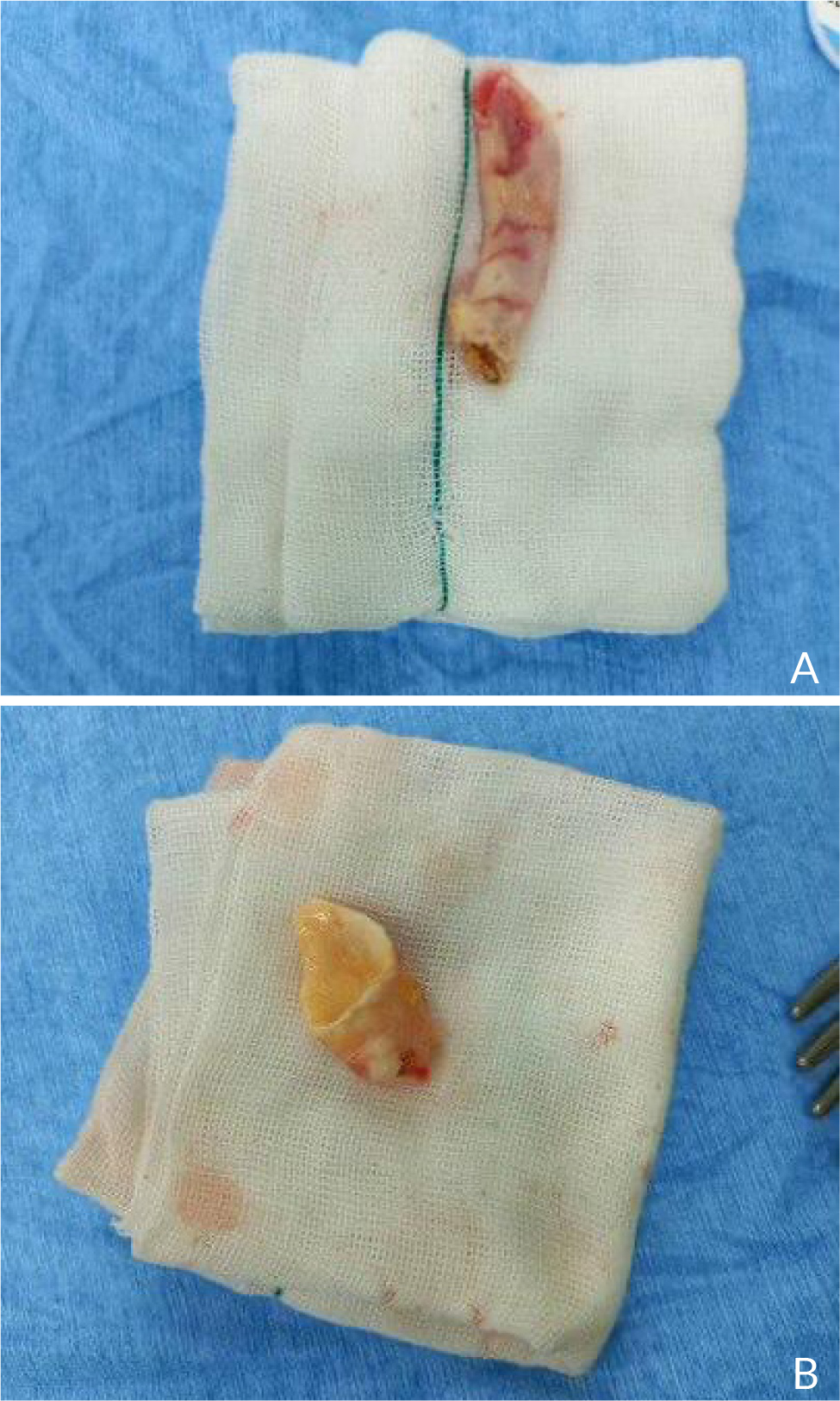
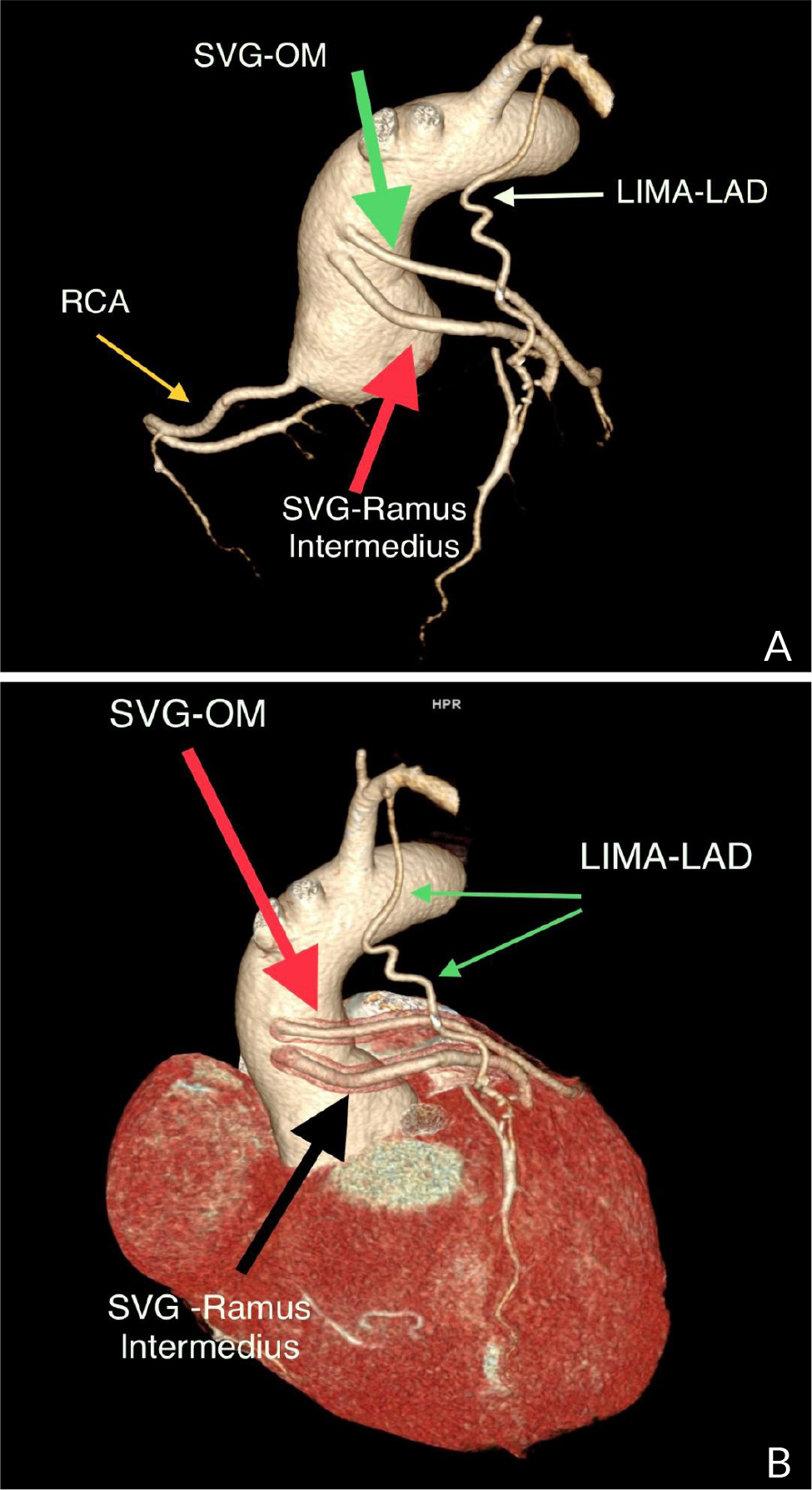
A 40-year-old female with no significant medical history presented to the outpatient cardiology clinic with recurrent episodes of anginal pain during the last year. There was no palpitation, and there was no history of syncope or dyspnea. There was no family history of cardiac disease or sudden cardiac death. She denied smoking and alcohol consumption and had a negative history of allergies. Cardiovascular examination was remarkable for the continuous systolic-diastolic murmur of grade 3 intensity best heard in the second intercostal space. The rest of the examination was normal. Her resting electrocardiogram was normal. Transthoracic echocardiography showed mildly enlarged left ventricle with preserved systolic function and no left ventricular hypertrophy. Dilated left aortic sinus, left main (LM) coronary artery, and left circumflex artery (LCX) were noted on the left parasternal long axis view and short axis view on the level of the aortic valve, with a positive flow on color Doppler (Fig. 1 A, B, C, and D). On modified four-chamber apical view, a track of dilated artery that terminated into the right atrium (RA) was found, with a continuous turbulent systolic and diastolic flow noted on color Doppler (Fig.1E and F, Clip 1). The findings were consistent with an anomalous CCF. Computed tomography coronary angiography (CTCA) showed dilated LM and LCX coronary arteries. The dilated LCX was directed posteriorly toward the RA with an aneurysmal dilatation in its distal part before opening into the right atrium (Fig. 2). Three-dimensional reconstruction images of computed tomography angiography showed the opening site of the fistula into the RA preceded by aneurysmal dilatation of the distal part of LCX (Fig. 3). CTCA imaging of the CCF between LCX and RA confirmed no evidence of obstructive coronary arteries. Since the patient was symptomatic, surgical treatment was offered. The opening of the fistula at RA was closed from the right atrial side (Fig. 4). The aneurysmal part of LCX was ligated and the dilated track of LCX was resected. The dilated part of the LM coronary artery was also resected (Fig. 5). To provide coronary perfusion, coronary artery bypass grafting (CABG) was performed: left internal mammary artery (LIMA) to left anterior descending (LAD) artery and two venous grafts—one to obtuse marginal (OM) artery and one for ramus intermedius (Fig. 6). The postoperative period was uneventful, and the patient was discharged on the sixth day in stable condition. The patient came for a follow-up in the outpatient clinic after four weeks in good condition with no more episodes of anginal pain.
Figure 1. A) Transthoracic echocardiography in long-axis view shows dilated left circumflex artery and a B) flow inside on color Doppler. C) Short axis view on the level of aortic valve and coronary ostia shows dilated left aortic sinus and left main coronary artery and D) on color Doppler a flow seen inside dilated left circumflex artery (LCX). E) Modified apical 4 chamber view shows the track of dilated LCX terminated in right atrium (RA) with F) color Doppler flow directed toward RA demonstrating the coronary cameral fistula.
Clip 1.
Figure 2. Coronary computed tomography angiography of the coronary arteries using maximum intensity projection images shows dilated left main (LM) coronary artery (thin red arrow) with dilated left circumflex (LCX) artery (green arrow) that directed toward right atrium (RA). An aneurysmal formation of distal LCX seen before opening into RA (thick red and white arrows). Abbreviations: descending aorta, DA; right aortic sinus, RAS; non-coronary sinus, NCS; left aortic sinus, LAS.
Figure 3. Three-dimensional volume rendered reconstruction images of cardiac computed tomography angiography showing dilated both left main (LM) and left circumflex (LCX) coronary artery with aneurysmal part of LCX before opening into right atrium (RA) – LCX-RA fistula.
Figure 4. The opening of fistula into the right atrium during operative treatment (black arrow).
Figure 5. A) Resected track of dilated left circumflex artery. B) Resected part of left main coronary artery.
Figure 6. Reconstructed computed tomography angiography (volume rendering technique images) after the surgical repair of the coronary fistula and coronary artery bypass grafting. Abbreviations: saphenous venous graft to obtuse marginal branch, SVG-OM; saphenous venous graft to ramus intermedius artery, SVG- ramus intermedius; left internal mammary artery to left anterior descending artery, LIMA-LAD.
DISCUSSION
CCF, a rare condition, is defined as an abnormal connection between a coronary artery and a cardiac chamber, whereas a coronary arteriovenous fistula is a connection between the coronary artery with any segment of the systemic or pulmonary circulation[1]. CCFs can be subdivided anatomically based on the type of connection between the cardiac chamber and the coronary artery: a direct connection is called an arterioluminal fistula, and a connection via a sinusoidal network is called an arterio-sinusoidal fistula. The majority of coronary arteries (90 %) drain into the right-sided chamber or great vessels, while drainage into left-sided chambers is considered very rare[2]. CCF may be single or multiple and can appear between one or more coronary arteries and a cardiac chamber. This anomaly originates in approximately half of the cases (55%) from the right coronary system, whereas 35% originate from the left-sided system, and in only 5% of cases, the origin site is bilateral[2]. CCF has no gender or race predominance and is found in less than 1% of the population. Only 0.1% to 0.2% of cases are detected during coronary angiographic studies[3]. A diagnosis of CCF can be made at any age. Incidental findings of heart murmur in childhood can lead to the diagnosis of CCF. Most CCFs remain asymptomatic and hemodynamically insignificant, especially if the size of the shunt is small and not compromising coronary blood flow. The mechanism through which the ischemia may occur distal to the fistula is a steal phenomenon that may manifest clinically as anginal pain, especially during exercise, in adulthood or during feeding in infants. The presence of the steal phenomenon is considered a sign of hemodynamically significant consequences of fistula, as reported by Angelini et al.[4].
The severity of the fistula is usually determined by the rate of blood flow between the nutrient vessel and the fistula. Periods of hypotension may result in flow diversion into the track of the fistula with subsequent low flow to the distal nutrient vessel resulting in ischemia; however, hypertension keeps the flow in the nutrient vessel satisfactory and well tolerated[5]. Another clinical manifestation of hemodynamic fistula with a different spectrum of symptoms is heart failure. Since the majority of CCFs are hemodynamically insignificant and clinically asymptomatic, the majority of CCFs are discovered incidentally when imaging is used for other clinical purposes. Despite coronary angiography being considered the gold standard to diagnose CCF, several other noninvasive tests are also available to detect and assess the anatomical and functional status of fistulas[6]. These noninvasive methods include conventional echocardiography and transesophageal approach[7], multidetector computed tomography[8], and cardiovascular magnetic resonance imaging[9]. Computed tomography coronary angiography is the noninvasive technique of choice to determine anomalous coronary artery anatomy with high accuracy. It allows to clearly delineate the cardiac chambers and the course of coronary arteries with their site of origin and termination and is considered suitable for patients with suspected coronary artery fistula.
There is no consensus on the management of symptomatic CCFs. Based on the size and volume of the left-to-right shunt and the functional status of the fistula, the current therapeutic strategies include watchful waiting and close follow-up, conservative medical management, percutaneous transcatheter embolization (PTE) and surgical closure.
Wang et al. demonstrated a highly success rate of surgical closure of fistulas under cardiopulmonary bypass without residual shunt, with no mortality or significant morbidity recorded[10]. Alekyan et al. reported a 93% success rate of PTE in a series of 15 patients with one early death and no recurrence after a follow-up period of up to 13 years[11]. The fistula closure method is indicated in cases of complex fistula anatomy in the presence of other comorbidities[12]. In a Dutch study, the characteristics of the fistula determined the treatment method: PTE was a preferred method of closure in cases of anatomically proximal CCF with termination away from the normal coronary artery in older individuals, while surgical ligation was more suitable for individuals with larger fistulas and multiple fistulas associated with cardiac disease.
CONCLUSION
Coronary cameral fistula is a rare pathology that can clinically manifest as angina. Computed tomography coronary angiography is a highly accurate diagnostic modality. Surgical treatment may be indicated to improve symptoms.