ABSTRACT
Drug-induced liver injury (DILI) is a liver condition caused by any drug or toxic substance. The spectrum of DILI manifestations can range from asymptomatic elevation of liver enzymes to acute liver failure. Antibiotics are one of the major causes of DILI. The authors report the case of a 37-year-old male with nausea, right hypochondrium pain and fever with history of bismuth subcitrate, metronidazole and tetracycline combination treatment in the previous five days. DILI was suspected and other aetiologies of acute hepatitis were excluded such as viral, autoimmune or even haemochromatosis and Wilson’s disease. Liver biopsy was performed, being compatible with the diagnosis of DILI; DILI is a diagnosis of exclusion. Bismuth subcitrate, metronidazole and tetracycline combination treatment is a rare cause of DILI.
LEARNING POINTS
- Bismuth subcitrate, metronidazole and tetracycline in combination is a rare cause of drug-induced liver injury (DILI).
- DILI is an exclusion diagnosis.
- Liver biopsy has an important role in the diagnosis of DILI.
KEYWORDS
Bismuth subcitrate, metronidazole, tetracycline, drug-induced liver injury
INTRODUCTION
Drug-induced liver injury (DILI) is a condition defined by any degree of liver injury caused by any drug or toxic substance and is the most common cause of acute liver failure in the Western world[1].
Given that it is a diagnosis of exclusion, without specific diagnostic tests and a low rate of reporting to pharmacovigilance systems, it is difficult to estimate the incidence of DILI, but some studies estimate between 14 and 19 cases per 100,000 population[2].
DILI encompasses a wide spectrum of manifestations from asymptomatic elevation of liver enzymes to acute liver failure, and can mimic viral hepatitis and other types of liver diseases[1,2]. Antibiotics are the most common cause of drug-induced liver injury[3].
Bismuth subcitrate, metronidazole and tetracycline is a drug combination used to treat Helicobacter pylori (HP) infection[4]. It is a combination of antibiotics that was proved to be effective and safe against HP, associated with a proton pump inhibitor over 14 days. HP eradication is important since it is associated with gastric and duodenal ulcers, and a risk factor for gastric cancer[4].
CASE DESCRIPTION
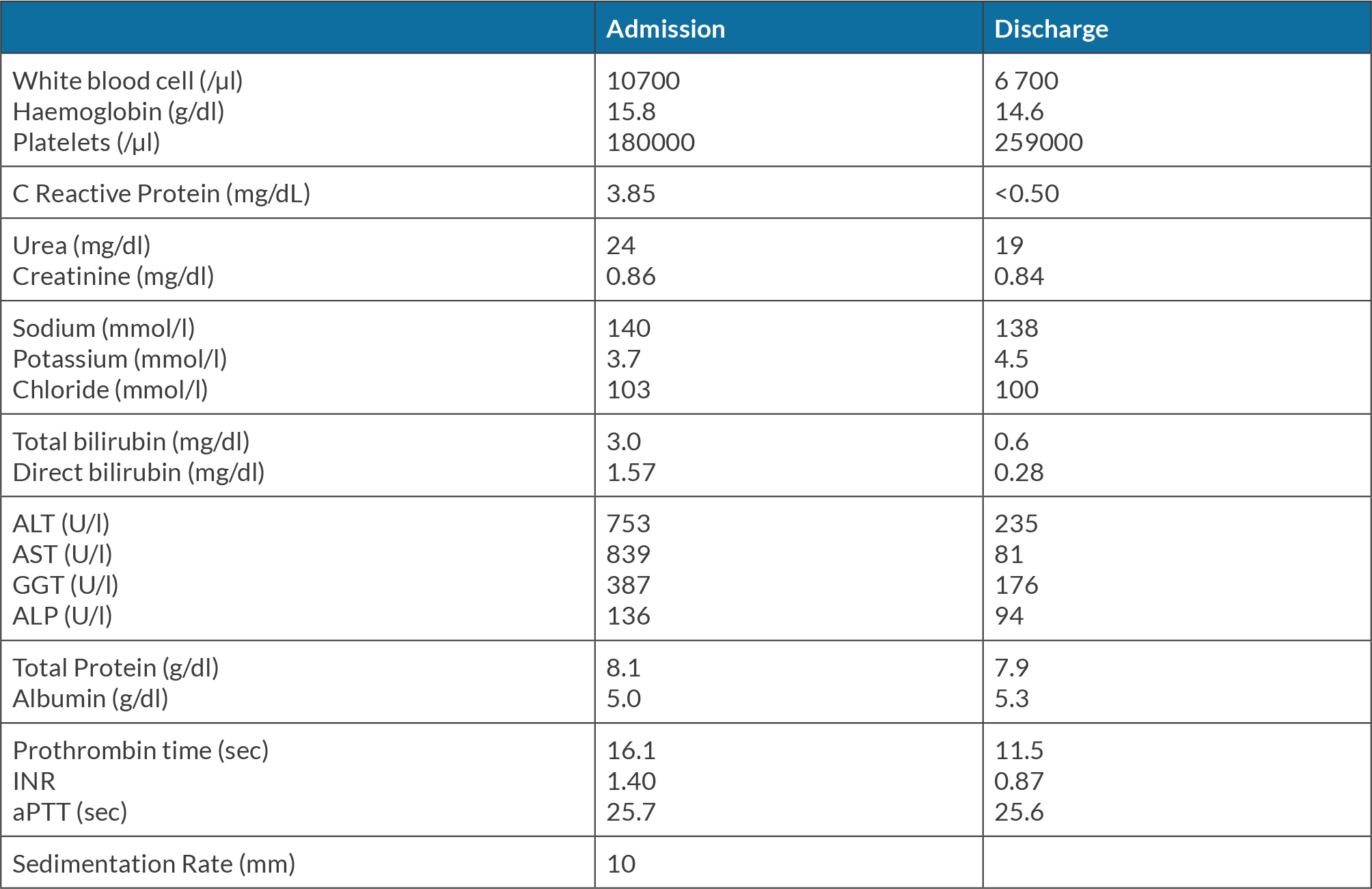
The authors present the case of a 37-year-old man, whose significant past medical history included asthma and a recent diagnosis of HP infection after two months of dyspepsia, which was medicated with bismuth subcitrate, metronidazole and tetracycline combination. Five days after initiating this medication, the patient demonstrated nausea, right hypochondrium pain and fever. By day six it had become worse, so he went to the emergency room. He denied recent travels, sick contacts or any recent alcohol abuse. He also denied ingestion of herbal remedies or illicit drugs. There was no evidence of jaundice, organomegaly, flapping or rash. Laboratory evaluation revealed a white blood cell count of 10700/μl, haemoglobin of 15.8 g/dl, platelets of 180,000/μl, C-reactive protein (CRP) 3.85 mg/dl, with normal renal function and electrolytes. His hepatic panel revealed an alanine aminotransferase (ALT) of 753 U/l, aspartate aminotransferase (AST) 839 U/l, total bilirubin 3.0 mg/dl, direct bilirubin 1.57 mg/dl, alkaline phosphatase (ALP) 136 U/l, gamma glutamyl transferase (GGT) 387 U/l, total protein 8.1 g/dl, albumin 5.0 g/dl and INR 1.40 (Table 1); his prior liver function tests were within normal limits.
A liver ultrasound revealed hepatic steatosis with normal biliary tree and portal vein patency. Abdominal tomography showed a liver with normal dimensions and homogeneous density. This patient’s initial treatment consisted of fluids and N-acetylcysteine infusion. Initially he presented clinical and analytical improvement but on day four after admission he presented a new elevation of hepatic enzymes. We intensified fluid therapy and there was marked clinical improvement until his discharge (Table 1).
From the aetiological study: viral hepatitis serologies including hepatitis A, B, C and E serologies as well as cytomegalovirus (CMV), Epstein-Barr virus (EBV), human immunodeficiency virus (HIV) and herpes simplex virus (HSV) were negative. Mononucleosis and leptospirosis tests were also negative. Wilson’s disease and haemochromatosis were excluded. Anti-nuclear, anti-mitochondrial and anti-smooth muscle antibodies were negative.
A ultrasound-guided percutaneous liver biopsy was performed only 4 weeks after the onset of the symptoms, as this technique was not accessible earlier in our hospital and also because the patient had a favourable evolution. It revealed hepatic parenchyma of preserved sinusoidal architecture with a normal relationship between the portal spaces and the centrilobular veins, without relevant alterations except for a slight mononuclear inflammatory infiltrate in the portal spaces; no signs of primitive or metastatic malignancy were observed.
All laboratory tests were normal 4 weeks after stopping bismuth subcitrate, metronidazole and tetracycline. He was transferred to Gastroenterology to decide on another eradication treatment for HP infection.
DISCUSSION
In any case of acute hepatitis it is important to take a careful medical history to identify changes in medication including type, dosing and duration of use. In this case, the time elapsed between the start of treatment with bismuth subcitrate, metronidazole and tetracycline and the occurrence of symptoms brought the hypothesis of DILI to a higher level. Hepatocellular reactions are the most common type of DILI, presented with a disproportionate elevation in AST and ALT compared with ALP[1].
As DILI is a diagnosis of exclusion, it was then important to exclude other aetiologies of acute hepatitis such as viral, autoimmune or even haemochromatosis and Wilson’s disease. Despite the pattern of liver injury being hepatocellular, biliary or venous outflow obstruction was also excluded.
The Roussel Uclaf Causality Assessment Method (RUCAM) is a system that assigns points for clinical and analytical features to assess the probability of DILI[5]. The RUCAM score in our case was 7, which means DILI was ‘probable’. The Naranjo score is used to assess the probability that a drug caused an adverse reaction[6]. In our case this score was 6, which means ‘probable’.
Liver biopsy is not mandatory for the diagnosis of DILI, but in our case it was only possible 4 weeks after the onset of the clinical condition, and it still revealed portal inflammatory infiltrates. This is characteristic of autoimmune hepatitis, but is also compatible with the diagnosis of DILI[7]. Considering the fact that the aetiological study was negative, (including the autoimmune study), recent drug exposure and analytical normalisation after a few weeks of drug cessation, DILI was assumed as the most likely diagnosis.
Therefore, in situations where the diagnosis or the severity is uncertain, a liver biopsy should be performed, possibly a transjugular approach in the presence of coagulopathy[7].
Bismuth subcitrate, metronidazole and tetracycline is a pill that contains three different drugs. To our knowledge there is no case report for DILI secondary to this drug combination; however, metronidazole and tetracycline were reported separately[5,8]. The cause of liver injury in the case of metronidazole is probably immunoallergic and, despite being rare it can result in liver failure and death[8]. For tetracycline it is more ambiguous, but tends to result in a cholestatic liver injury[5]. In our case, there was a hepatocellular injury which, with support treatment and drug suspension, led to clinical improvement.
CONCLUSION
After all the results and according to the drug history, bismuth subcitrate, metronidazole and tetracycline appeared to be the most likely causative agent, and our presumed diagnosis was DILI. It is important not to forget that drug exposure preceding the onset of liver injury, excluding other causes of liver diseases and drug discontinuation that leads to improvement in the liver injury, are the key elements for the diagnosis of DILI.