ABSTRACT
Introduction: Dysphagia in post COVID-19 patients could be caused by several factors, including reduced pharyngolaryngeal coordination due to SARS-CoV-2 tropism to the central and/or peripheral nervous system. To our knowledge, this is the first reported case of COVID-19-related dysphagia successfully treated with botulinum toxin type A injection.
Case description: We report the case of a patient with severe oropharyngeal dysphagia due to COVID-19 confirmed by fibre endoscopy. As a result, the patient required an enteral feeding tube. After two months of traditional swallowing therapies, there was only limited improvement. An electrophysiologic evaluation of the cricopharyngeal muscle was performed and showed a normal inhibition of the cricopharyngeal muscle, followed by a hypertonic rebound. Based on this result, we decided to perform a unilateral laryngeal injection of botulinum toxin type A. After the injection, the patient’s swallowing function improved significantly, allowing him to return to oral feeding.
Discussion: Newly diagnosed oropharyngeal dysphagia was found in 35.3% of hospitalised patients with COVID-19. There are several possible causes of COVID-19-associated dysphagia, including stroke, encephalitis, critical illness neuropathy, Guillain-Barré syndrome and skeletal muscle injury. In our case, since stroke was excluded by brain MRI, cranial nerve injury was a possible explanation for the difficult recovery of swallowing despite daily swallowing therapy.
Conclusion: We suggest that electrophysiology is a valid tool for the diagnosis and follow-up of patients with oropharyngeal dysphagia.
LEARNING POINTS
- SARS-CoV-2 tropism to the central and/or peripheral nervous system can cause dysphagia in post COVID-19 patients.
- An electrophysiologic approach is useful for the diagnosis and follow-up of patients with oropharyngeal dysphagia.
- A single botulinum toxin type A injection is a valid treatment option to improve the swallowing function in patients with post COVID-19 dysphagia.
KEYWORDS
COVID-19, dysphagia, botulinum toxin, EMG.
INTRODUCTION
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused a human infection (COVID-19) that originated in China and quickly became a public and international health emergency. The clinical spectrum of COVID-19 ranges from asymptomatic or paucisymptomatic patients to septic shock and multi-organ failure. The most common symptoms include fever, a dry cough, malaise, dyspnoea, myalgia, and loss of smell and taste. Cases of greater severity may progress to acute respiratory distress syndrome, multi-organ failure and/or neurologic sequelae[1]. A significant percentage (9–32%) of COVID-19 patients require admission to an intensive care unit (ICU)[2]. The prolonged duration of mechanical ventilation, combined with sedation and bed rest, is associated with dysphagia due to altered laryngeal sensitivity, as evidenced by the absence of the laryngeal adductor reflex in approximately half the patients[3]. Consequently, there is a high risk of dysfunction of swallowing mechanisms that may persist for months or years after discharge from the ICU[2,4].
The act of swallowing is coordinated and executed by a widely distributed network involving cortical, subcortical and brainstem structures as well as downstream peripheral nerves and muscles. Complications of COVID-19 may also affect this network at different levels, exacerbating dysphagia in critically ill patients[5].
Botulinum toxin type A (BoNT-A) is very effective in reducing excessive activation of striated muscle and is therefore widely used in many neurological disorders characterised by excessive muscle contraction. Most previous studies indicate that neurogenic dysphagia associated with upper oesophageal sphincter (UES) spasm or dyskinesia can be effectively treated by injection of BoNT-A into the cricopharyngeal muscle[6].
To the best of our knowledge, this is the first study to evaluate the efficacy of BoNT-A injection for neurogenic dysphagia with UES involvement in post COVID-19 patients. Within the limitations of a single-case study, our result may suggest that BoNT-A injection represents a valid approach as a rehabilitative treatment for these subjects.
CASE DESCRIPTION
We discuss the case of a 61-year-old man with no family history of genetic and metabolic diseases. The patient had no significant medical or surgical history, and no previously documented dysphagia. After testing positive for COVID-19, he was admitted to the ICU with pneumonia and mild respiratory insufficiency and treated with helmet non-invasive ventilation (FiO2 65%) for two days. Due to the persistence of severe respiratory dysfunction, the patient was intubated and placed in the prone position. During the recovery period, he presented with haemodynamic instability due to concurrent Enterococcus faecalis infection, periodic atrial tachycardia treated with intravenous amiodarone infusion and continuous renal replacement therapy.
One month after admission to the ICU, the patient could be extubated due to improved respiratory status and negative PCR for SARS-CoV-2.
The patient presented with severe muscle hypotrophy and neurological examination revealed a remarkable loss of strength, especially in the proximal sectors of the upper limbs and distal sectors of the lower limbs, and osteotendinous reflexes (ROTs) were no longer elicited in the lower limbs. After neurophysiological evaluation, critical illness myopathy and/or neuromyopathy (CRIMYNE) was diagnosed.
The patient received rehabilitative swallowing therapies with limited improvement, therefore a fibreoptic endoscopic evaluation of swallowing (FEES) test was performed. The instrumental evaluation showed a paresis of the left vocal cord and the presence of saliva in the laryngeal vestibule. Furthermore, pre-glutitive inhalation before the pharyngeal phase or before the opening of the upper oesophageal sphincter (UES) with both liquid and solid bolus confirmed the diagnosis of severe dysphagia (DOSS=1/7). DOSS – the dysphagia outcome and severity scale – is a 7-point scale designed to easily assess the functional severity of dysphagia based on objective assessment, and to provide recommendations for dietary level, level of independence and type of diet. In addition, a percutaneous endoscopic gastrostomy (PEG) tube was placed to meet the patient’s nutritional needs.
The patient also underwent a brain MRI, which showed no vascular or inflammatory lesions. After another four weeks, he was transferred to our neuro-rehabilitation unit for intensive rehabilitation. While the patient showed improvement in muscle strength and functional ability, the dysphagia persisted despite daily swallowing therapy training, which included effortful swallowing, shaker exercises, chin tuck against resistance and expiratory muscle strengthening.
Twelve days after admission to the neuro-rehabilitation unit, an additional FEES test was performed, which showed the presence of slight residues in the valleculae of extremely thick drinks administered under observation and thick saliva over the vocal cords. The residues were removed with an effective cough.
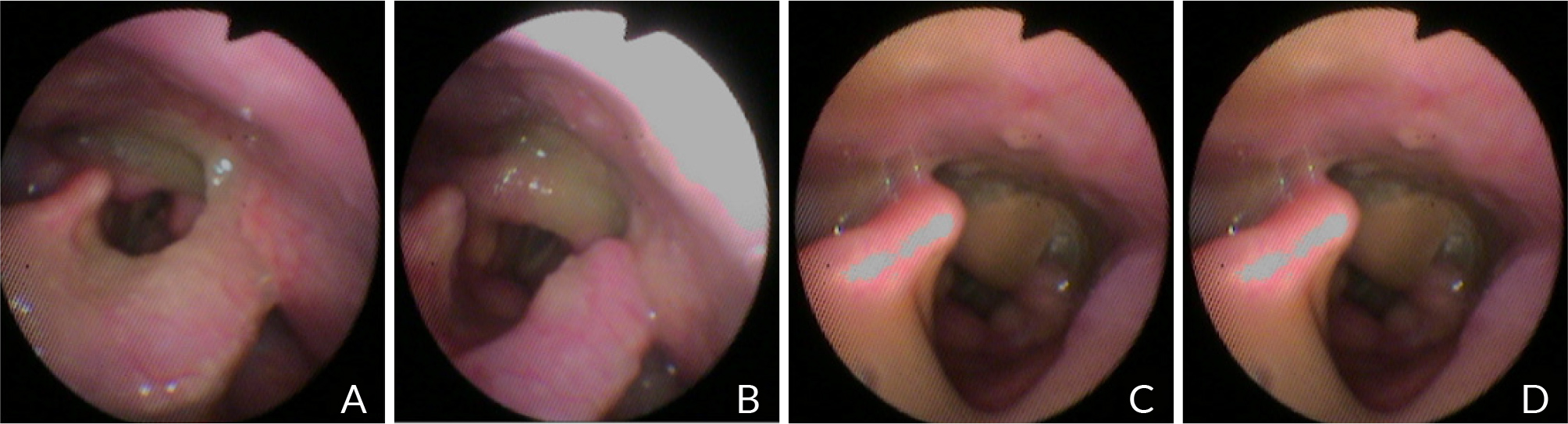
Different textures (thin and extremely thick drinks, pureed food) were administered to the patient. From the video endoscopic view, the examiner noticed a very weak basal lingua retropulsion, the presence of residues in the two valleculae and the two pyriform sinuses, and the most significant retro-cricoid residues with difficulty in clearing from the pharynx (Fig. 1).
Figure 1. A, B) Residual is visible in post pharyngeal swallow on the epiglottis and in the retro-cricoid region. C, D) Residual enters the airway, remains above the vocal folds and is not completely ejected from the airway.
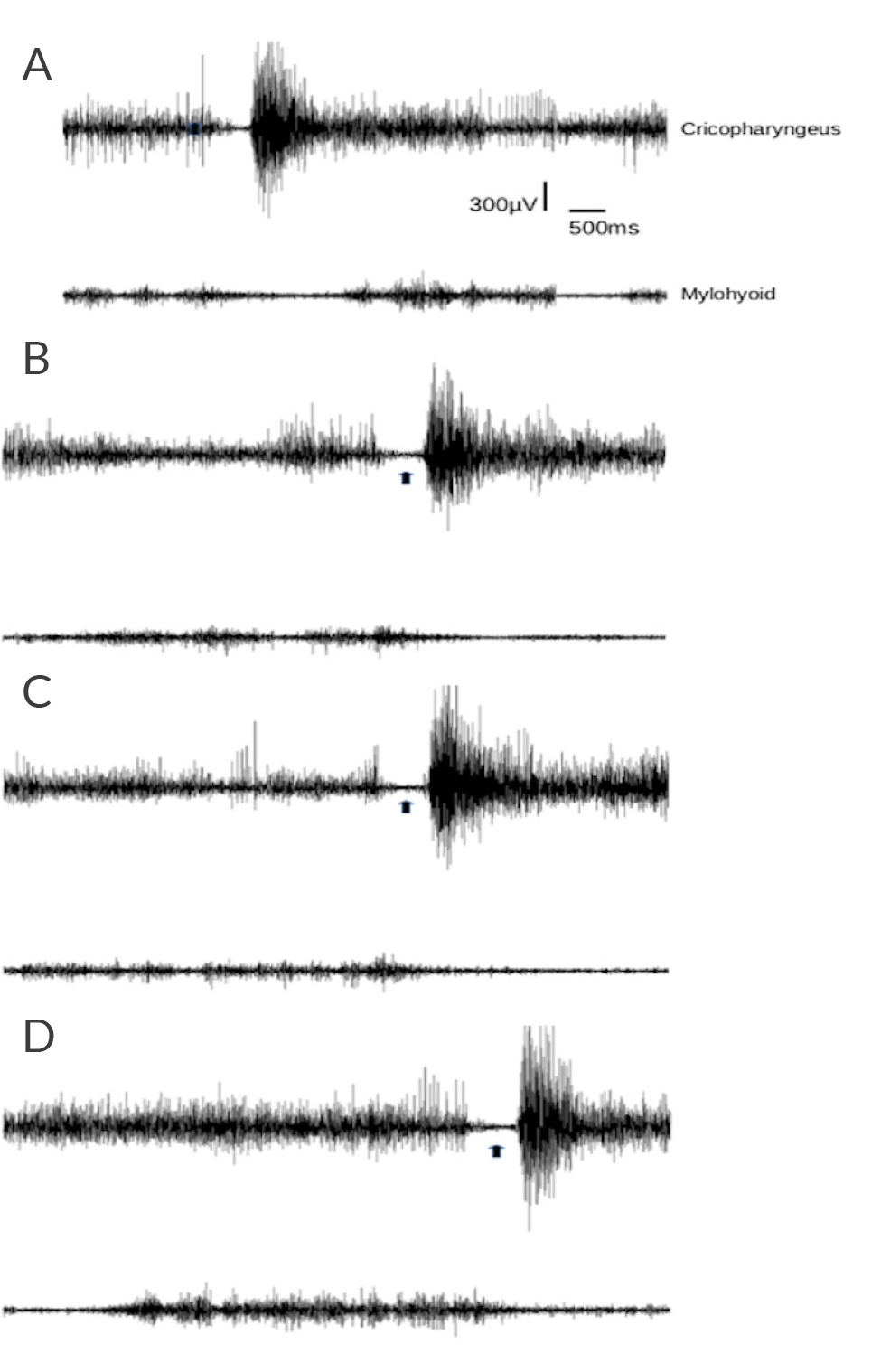
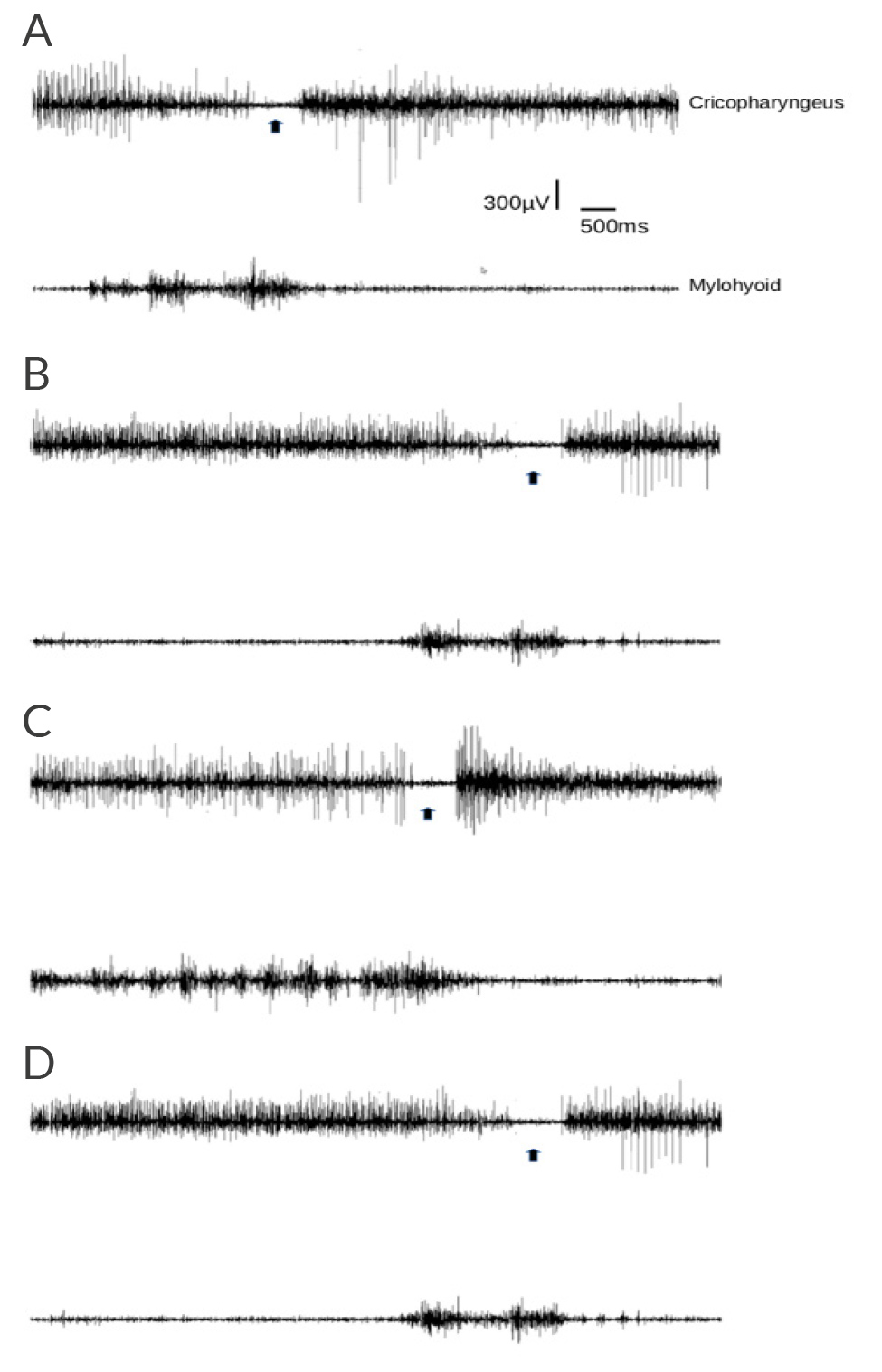
To complete the dysphagia studies, an electromyography (EMG) of the cricopharyngeal muscles was performed, which showed normal inhibition during the initial phases of swallowing, but abnormally increased EMG activity at the end. The suprahyoid/submental muscles showed prolonged activation before the start of swallowing, reflecting difficulties in the pre-swallowing phase (Fig. 2). An electromyography-guided injection of incobotulinum toxin A (Incobotulinum toxin A – Xeomin®, Merz, Frankfurt, Germany) into the right cricopharyngeal muscle was performed, which was well tolerated and without complications. Informed consent was obtained from the patient prior to the procedure and the tenets of the Declaration of Helsinki were followed. The protocol was approved by the Institutional Review Board.
Figure 2. Electromyographic recording from cricopharyngeus and mylohyoid muscles during swallowing before botulinum toxin treatment. The cricopharyngeus muscle was recorded using a monopolar teflon-coated hollow needle, while for the suprahyoid/submental muscles surface adhesive electrodes were placed over the skin of the suprahyoid/submental region bilaterally, at an interelectrode distance of 30 mm. The black arrows indicate cricopharyngeus EMG activity inhibition corresponding to each swallowing attempt. Reproducibility of activation pattern can be appreciated across four repetitions (A to D).
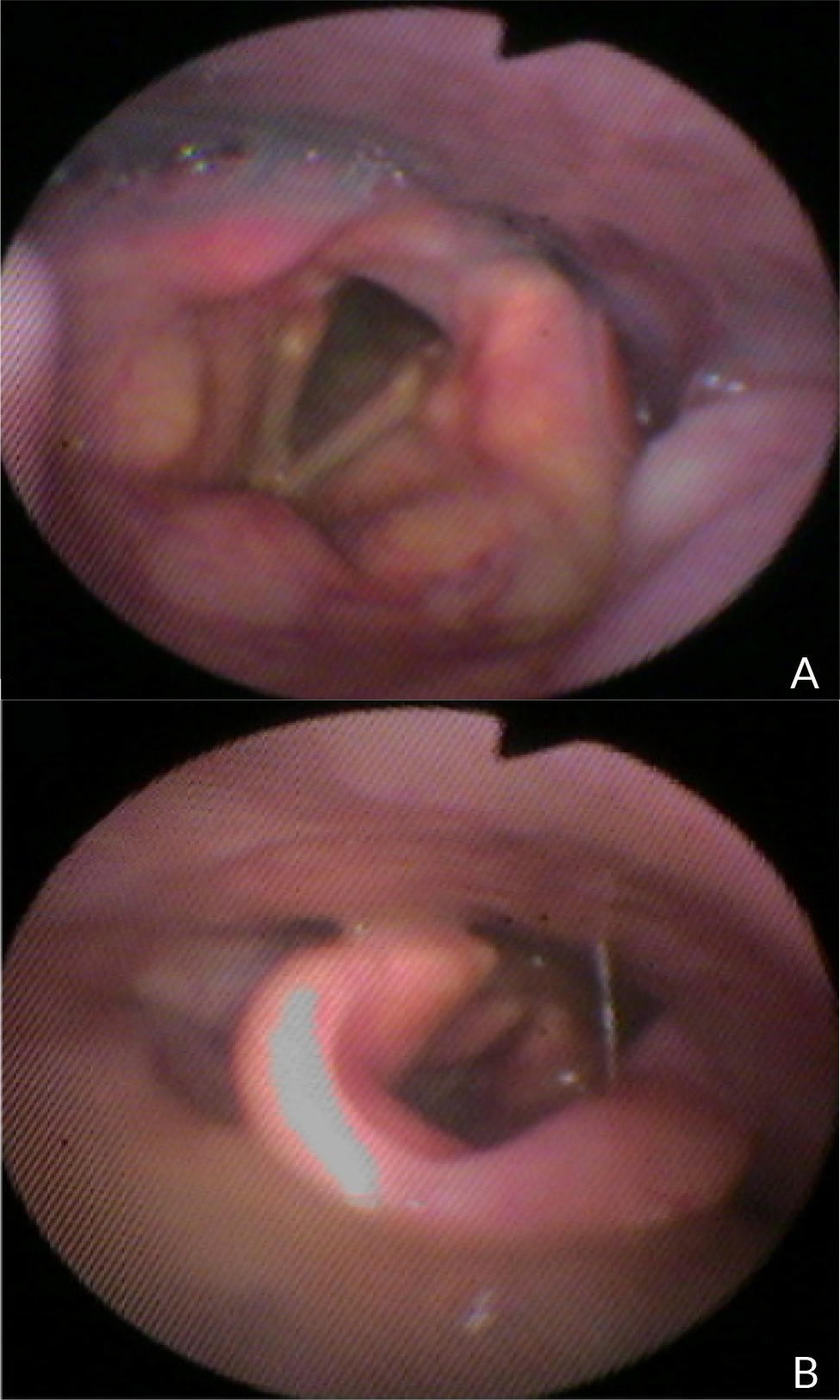
Fifteen days after the injection, a FEES test (Fig. 3) was repeated and showed an improvement in pharyngeal muscle coordination and less food residue (DOSS=4/7); consequently, the patient started to accept extremely thick drinks and pureed food.
Figure 3. A) Residues may be seen in pyriform right sinus, no residues remain above the vocal folds, opening of the glottis post-swallow inspiration, no stasis. B) Execution of pharyngeal swallow with rapid closure of the glottis.
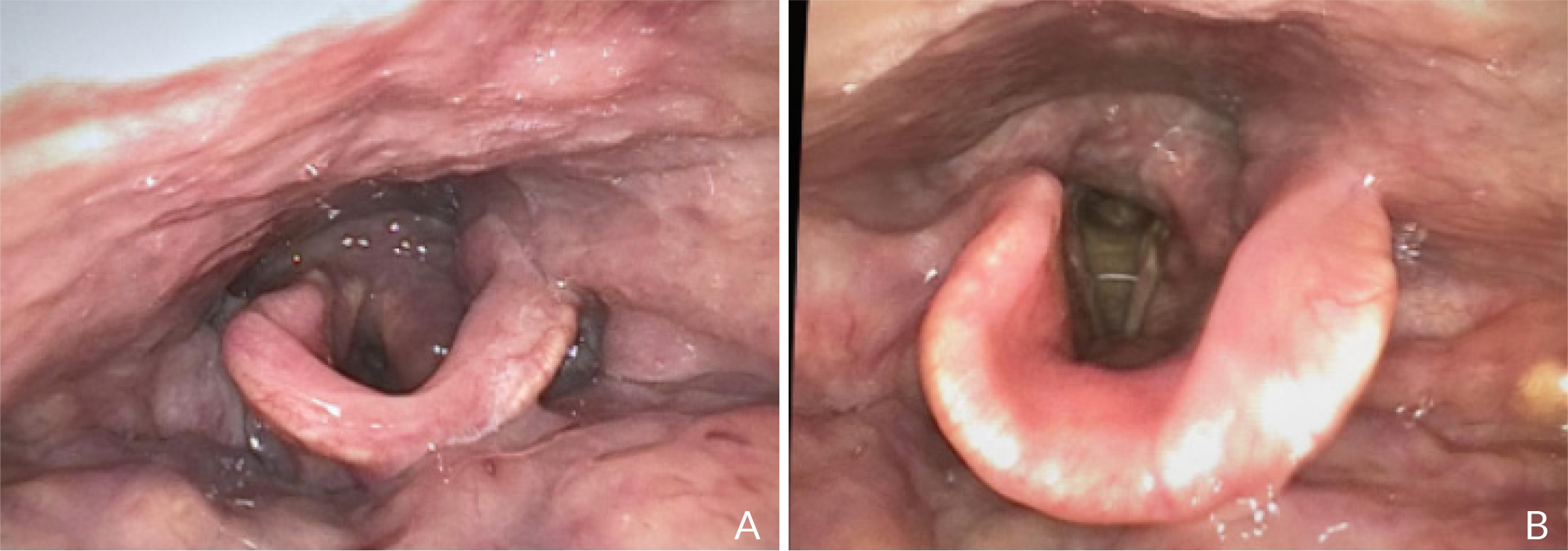
The last FEES test, performed 20 days after the previous one (Fig. 4), confirmed the favourable evolution of the swallowing pattern; the patient was able to properly remove food remnants on a second swallow and an efficient cough reflex was present. Forty-five days after the BoNT-A injection, the abnormal increase in EMG activity in the cricopharyngeal muscle after swallowing was no longer evident (Fig. 5).
Figure 4. A, B) One month after botulinum toxin treatment no food residues are visible after the administration of all kinds of textures.
Figure 5. Electromyographic recording from cricopharyngeus and mylohyoid muscles during swallowing after botulinum toxin treatment. The black arrows indicate cricopharyngeus EMG activity inhibition corresponding to each swallowing attempt. Reproducibility of activation pattern can be appreciated across four repetitions (A to D). EMG hyperactivity of cricopharyngeus muscle is clearly reduced after botulinum toxin treatment.
Since it was possible to provide adequate nutrition per OS the PEG tube was removed, and the patient was discharged on a standard diet with a preference for soft and bite-sized foods. One month after discharge, the patient reported no swallowing difficulties at the follow-up visit (DOSS=6/7).
DISCUSSION
The coronavirus enters the human body by binding to angiotensin-converting enzyme 2 cell receptors expressed in the tongue, oral mucosa and olfactory epithelium. Cranial nerves may be involved in COVID-19 by direct viral invasion, but the virus can also affect the central nervous system. In our patient, decreased laryngeal sensitivity after prolonged intubation combined with COVID-19 infection could be a manifestation of glossopharyngeal and vagal neuropathies. In addition, coughing, sneezing and shortness of breath may interfere with respiratory-swallowing coordination, which is essential to protect the lower airway and prevent aspiration pneumonia. A concurrent decline in physical function due to malnutrition and deconditioning from acute infection may also contribute to the development of dysphagia[8].
BoNT-A is already used with benefit in other types of neurological or non-neurological dysphagia associated with UES hyperactivity or reduced relaxation, with the advantages that it is repeatable, does not require general anaesthesia and can therefore be administered to debilitated patients[9].
We injected 30 U incobotulinum toxin A, diluted 5.0U/0.1 ml under ultrasound and electromyographic guidance on the right side only. The BoNT-A injection was performed unilaterally because bilateral injection could have increased the risk of oesophageal reflux and subsequent aspiration. Another reason for unilateral injection was to reduce the effects of potential toxin diffusion to nearby muscles involved in vocal cord movement (the posterior and lateral cricoarytenoid muscles) and pharyngeal peristalsis (the inferior constrictor muscle), as both the UES wall and its muscular layer are extremely thin[6,10].
The success of the procedure was comprehensively evaluated using the following criteria: clinical resolution of solid and liquid dysphagia, cough, choking, regurgitant aspiration and hypersalivation; DOSS for clinical evaluation of swallowing; FEES for evaluation of reduced retention of remnants in the laryngeal vestibule.
No adverse events were reported, including transient chest pain, heartburn, pneumothorax, mediastinitis, gastroparesis and aspiration, and because the patient showed significant improvement in all of these parameters, no additional injections were required.
CONCLUSION
Cricopharyngeal muscle injection with botulinum toxin under electromyographic guidance may be a rapid, repeatable and useful choice for the treatment of UES dysfunction in patients with post-ICU-acquired dysphagia with concomitant COVID-19 infection. We also suggest that laryngeal injection of botulinum toxin type A is a feasible option to restore safe swallowing function in patients with cricopharyngeal hypertonia due to COVID-19.