ABSTRACT
Background: Castleman disease (CD) is a rare lymphoproliferative disorder with various subtypes, including the HHV-8-negative/idiopathic multicentric CD (iMCD). The diagnosis of iMCD remains challenging due to its non-specific presentation, in the form of generalised lymphadenopathies and inflammation. Two clinical presentations have been recently defined: a severe form iMCD-TAFRO and a milder form of iMCD not otherwise specified (iMCD-NOS). identification of interleukin-6 (IL-6) as a major culprit of inflammatory symptoms led to the development of anti-IL-6 therapies, with siltuximab being the approved first-line treatment.
Case description: A 16-year-old male presented with recurrent fever, night sweats and several other non-specific symptoms. After extensive evaluations, an excisional lymph node biopsy confirmed the iMCD-NOS diagnosis. The patient received high-dose steroid therapy followed by siltuximab for four years. This treatment was well tolerated with only mild neutropenia not leading to dose adjustment. On siltuximab, the patient developed two mild COVID-19 episodes. His response to siltuximab remained effective throughout four years.
Discussion: The absence of biomarker or causal agent identification poses a diagnostic challenge requiring lymph node histopathology for a definitive diagnosis of iMCD. Anti-IL 6 (siltuximab) is the recommended frontline therapy, suppressing inflammation and halting disease progression. Intravenous administration every 3 to 6 weeks can impact patient quality of life, prompting further research for alternative treatments. High-dose steroids, rituximab, cyclosporine, tacrolimus, lenalidomide or combined chemotherapy such as rituximab-bortezomib-dexamethasone are among the considered options according to disease severity.
Conclusion: Overall, long-term siltuximab effectively controlled iMCD symptoms and was well tolerated by this young adult, who endured two mild COVID-19 episodes.
LEARNING POINTS
- Lymph node biopsy rather than bone marrow biopsy is needed for the diagnosis of iMCD.
- We were able to control the patient's condition in the absence of cumulative toxicity during four years of siltuximab anti-IL6 therapy.
- Immunosuppressive anti-IL6 therapy did not worsen two episodes of COVID-19.
KEYWORDS
Lymphoproliferative disorders, multicentric Castleman disease, siltuximab, interleukin-6, COVID-19.
INTRODUCTION
Castleman disease (CD), a rare lymphoproliferative condition includes unicentric CD, human herpesvirus-8 (HHV-8)-associated multicentric CD and HHV-8-negative, idiopathic multicentric CD (iMCD) with unknown aetiology[1]. iMCD poses a diagnostic challenge due to the absence of known causative agents and its non-specific presentation with generalised lymphadenopathies and cytokine-driven inflammation that can lead to multi-organ failure. Diagnosis relies on lymph node (LN) histopathology in the absence of HHV-8.
In 2017, the Castleman Disease Collaborative Network (CDCN) established international diagnostic criteria for iMCD defining two clinical presentations: 1) iMCD-TAFRO, characterised by thrombocytopenia, anasarca, fever, reticulin fibrosis of bone marrow, renal insufficiency and organomegaly; 2) iMCD not otherwise specified (iMCD-NOS), which is a milder form with lymphadenopathies and hypergammaglobulinemia[2].
iMCD LN histopathology is classified into hyaline vascular/hypervascular, plasmacytic or mixed subtypes which share polyclonal hyperactivation in lymphoid tissues, increased mTOR signalling, an interferon signature and inflammatory cytokines production[3,4].
Among released inflammatory cytokines, interleukin 6 (IL-6) is a major contributor to symptoms, prompting the development of anti-IL-6 therapies. Two monoclonal antibodies have been developed: tocilizumab targets the IL-6 receptor while siltuximab targets IL-6 directly, the latter being the only drug approved in the United States, Canada and Europe for iMCD[3,5]. Due to immunosuppression, IL-6 blockade requires surveillance of infection severity, including COVID-19.
CASE DESCRIPTION
A 16-year-old male patient presented with recurrent episodes of fever, night sweats, headache, polyarthralgia and weight loss of 5 kg. His medical history included attention deficit hyperactivity disorder managed with oral methylphenidate (36 mg daily). His family medical history included a maternal grandfather with chronic leukaemia, a maternal aunt with Crohn's disease and rheumatoid arthritis, and his mother with psoriasis.
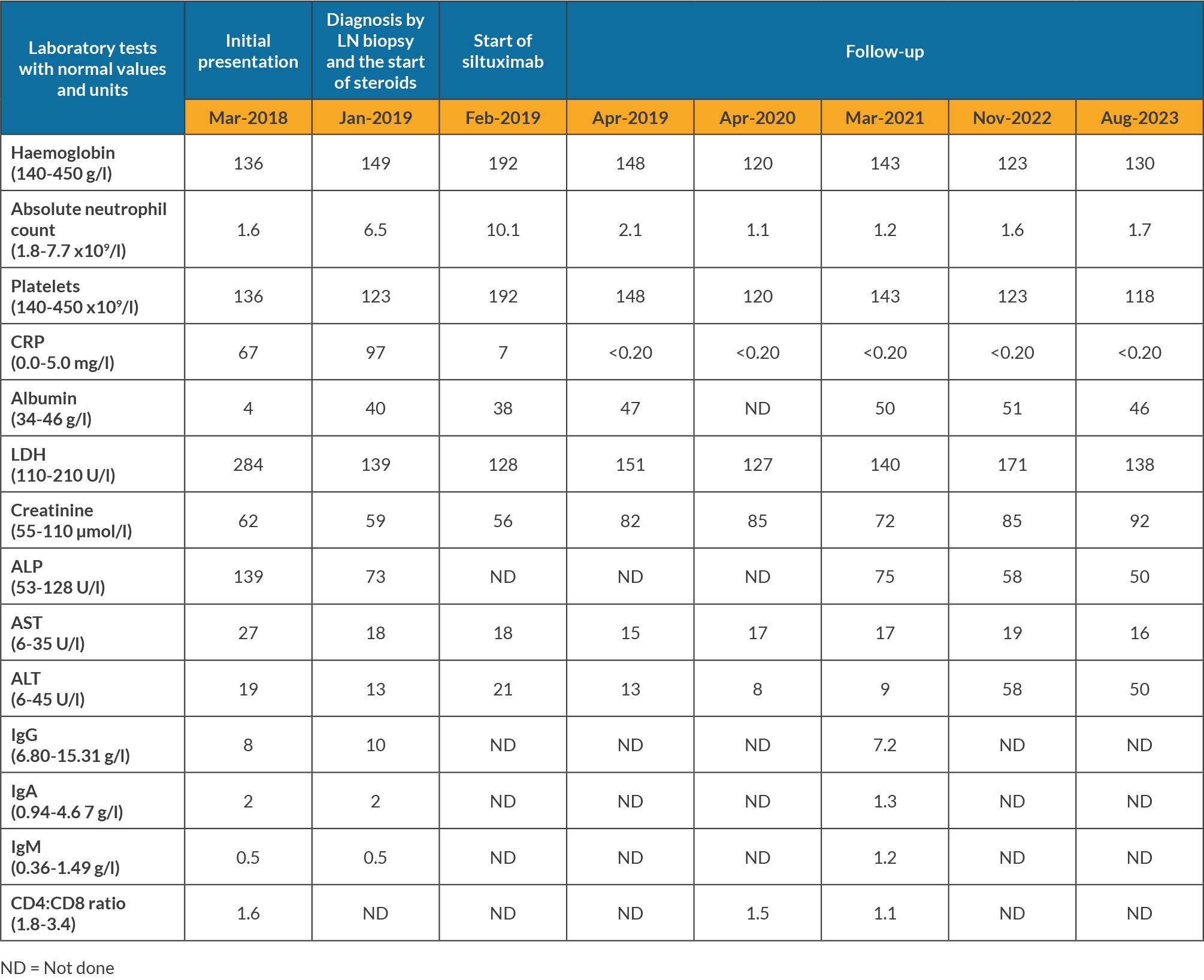
Blood tests showed intermittent mild neutropenia and thrombocytopenia, while liver and kidney function were normal (Table 1). Serology tests for viral hepatitis, HIV and HHV-8 were negative. Epstein Barr virus (EBV) IgG serology was positive and polymerase chain reaction was negative. Extensive autoantibody panel tests were negative.
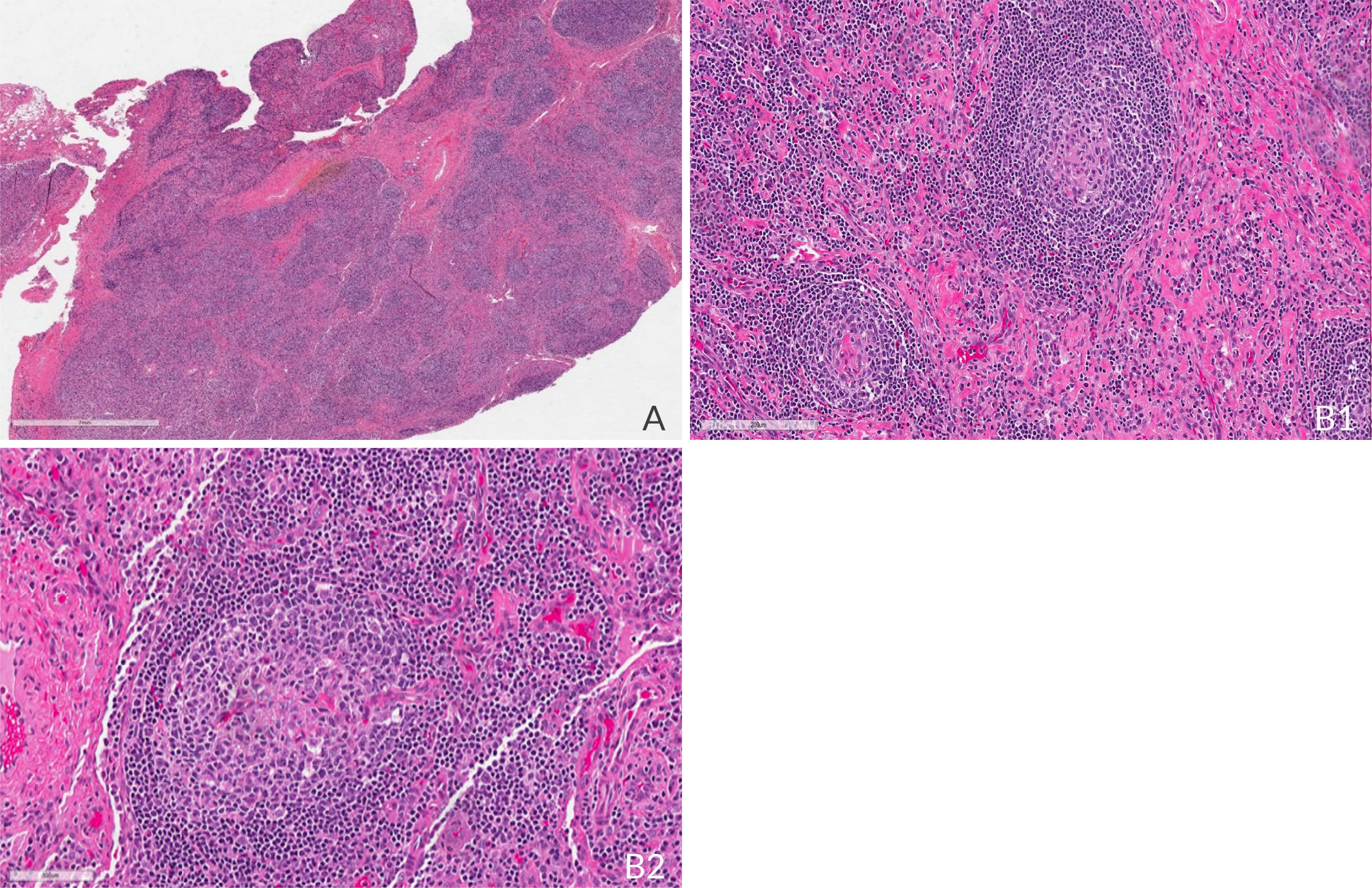
After consultations with infectious diseases, cardiology, rheumatology and ophthalmology teams over a year, tissue evaluation was needed for diagnosis. A bone marrow biopsy showed normal cellularity, maturation and differentiation in all cell lineages, with no signs of increased reticulin staining, fibrosis, granuloma, infection or malignancy. A positron emission tomography (PET) scan revealed hypermetabolic activity in LNs in the mediastinum, retroperitoneum, spleen and skeleton, with standardised uptake values ranging from 5.8 to 8.4. An excisional biopsy of the enlarged left cervical LN showed a diffuse proliferation of small uniform lymphocytes indicative of the hyaline vascular type of iMDC (Fig. 1). Immunohistochemistry staining demonstrated a normal IgG/IgG4 ratio, a normal kappa/lambda ratio, and no HHV-8 or EBV. Flow cytometry showed absence of monoclonal lymphocyte populations: 81% of cells were lymphocytes; 40% being T-cells with a CD4/CD8 ratio of 3.4/1.0, and 39% being B-cells with a normal kappa/lambda ratio. B-cells showed moderate expression of CD19 and CD20 and were negative for CD5. There was no evidence of lymphoma or other malignancy. These pathological findings and clinical presentation prompted the diagnosis of iMCD-NOS.
Figure 1. Left cervical LN (excisional biopsy). Haematoxylin eosin (H&E) showing histologic features of iMCD including A) capsular and interfollicular fibrosis, loss of normal nodal architecture, areas of atretic germinal centres with follicle 'twinning' (2×). At higher magnification, B1) follicles with characteristic concentric lymphocytic rimming, also called 'onion skinning', are seen (40×); B2) as well as interfollicular vascularisation, the so-called lollipop configuration where an aberrant vessel is seen entering the centre of an atretic germinal centre (40×).
One year after his first consultation, in January 2019 the patient received intravenous pulse methylprednisolone at 500 mg daily for three days, followed by oral prednisolone at 60 mg daily, tapered over four months. Four weeks later, he started intravenous siltuximab at 11 mg per kg every three weeks. Attempts to extend the dosing interval to four weeks were unsuccessful due to a recurrence of arthralgia a few days before the next dose. As of September 2023, the patient had received 77 cycles of siltuximab at three-week intervals. PET showed no sign of hypermetabolism in LN.
Four months post-siltuximab treatment, the patient developed mild neutropenia (1.1 to 1.6 cells/ml) without requiring dose adjustments. While on siltuximab, the patient tested positive for COVID-19 twice, in March and August 2022, experiencing mild symptoms without any impact on his underlying condition. Due to concerns about siltuximab's potential immunosuppressive effects, he received a five-day course of ritonavir-boosted nirmatrelvir (Paxlovid). These two onsets of COVID-19 occurred months after he received three doses of the Moderna mRNA COVID-19 (mRNA-1273) vaccine.
DISCUSSION
Idiopathic multicentric CD remains a diagnostic challenge as presentation is non-specific and can present concurrently with various infectious diseases including COVID-19, and malignant and autoimmune diseases. LN histopathological features in the absence of HHV-8 are considered diagnostic[6].
In 2018, the CDCN enhanced clinical awareness of the significance of pathology for disease diagnosis[6], and Van Rhee et al. presented an evidence-based treatment guideline with an algorithm based on disease severity[2]. For iMCD, siltuximab selectively targets IL-6, effectively suppresses inflammation, providing symptom relief and halting disease progression. Siltuximab is recommended as frontline therapy. Tocilizumab can be considered when siltuximab is unavailable, and tailored steroid doses can be used as adjunctive therapy. Responders should continue anti-IL-6 treatment long-term, given its good tolerance[5]. The recommended dose is 11 mg/kg every 3 weeks, with more frequent dosing if an accelerated response is needed. After an initial control, dosing intervals can be extended to every 6 weeks.
For severe cases or non-responders, patients may benefit from high-dose steroid, rituximab or cyclosporine, tacrolimus, lenalidomide, or combined chemotherapy such as R-CVP or R-CHOP[7].
Administering IL-6 blockade intravenously every 3 weeks negatively impacts a patient's quality of life. Moreover, cost remains a challenge, therefore alternative treatments are under evaluation. Yin et al. explored rituximab-bortezomib-dexamethasone in five patients as anti-IL-6 antibodies were not available[9]. Although patients achieved lasting responses for a median follow-up of 11 months after four cycles without requiring maintenance therapy, significant toxicities were observed[8].
Conversely to our patient, one severe case of COVID-19 was reported in a patient treated for iMCD with siltuximab[9]. Our patient experienced only mild symptoms during two COVID-19 episodes. This could be influenced by the patient's young age and prior COVID-19 vaccinations.
CONCLUSION
Globally, our 21-year-old patient achieved long-term tolerance to siltuximab with control of his symptoms related to iMCD[10,11], even during the main COVID-19 era[9,12].