ABSTRACT
Coronary artery fistulas (CAFs) are rare defects in the coronary circulation system that are usually diagnosed incidentally with cardiac imaging. Although the prognosis of coronary artery fistulas is highly variable, the complications to which they predispose patients are ultimately the determining factor. The authors describe a case of a 56-year-old male, a smoker, hospitalised for worsening dyspnoea on progressively smaller efforts, in the context of acute heart failure. During hospitalisation and imaging exams, a coronary-bronchial fistula was identified.
LEARNING POINTS
- CAFs are rare and may present with different anatomical configurations and clinical syndromes.
- Only 17% of CAFs reported draining into the pulmonary circulation.
- The optimal timing and role of CAFs intervention are not clearly defined.
KEYWORDS
Coronary artery fistula; coronary steal phenomenon; coronary to pulmonary artery fistula
INTRODUCTION
The impact of a CAF on heart function can vary depending on the size and location of the abnormal connection. If the fistula is small and does not cause significant shunting of blood away from the coronary circulation, it may not produce any noticeable symptoms or health issues. However, larger and more significant coronary artery fistulas can potentially lead to heart failure[1,3], as we describe below.
CASE DESCRIPTION
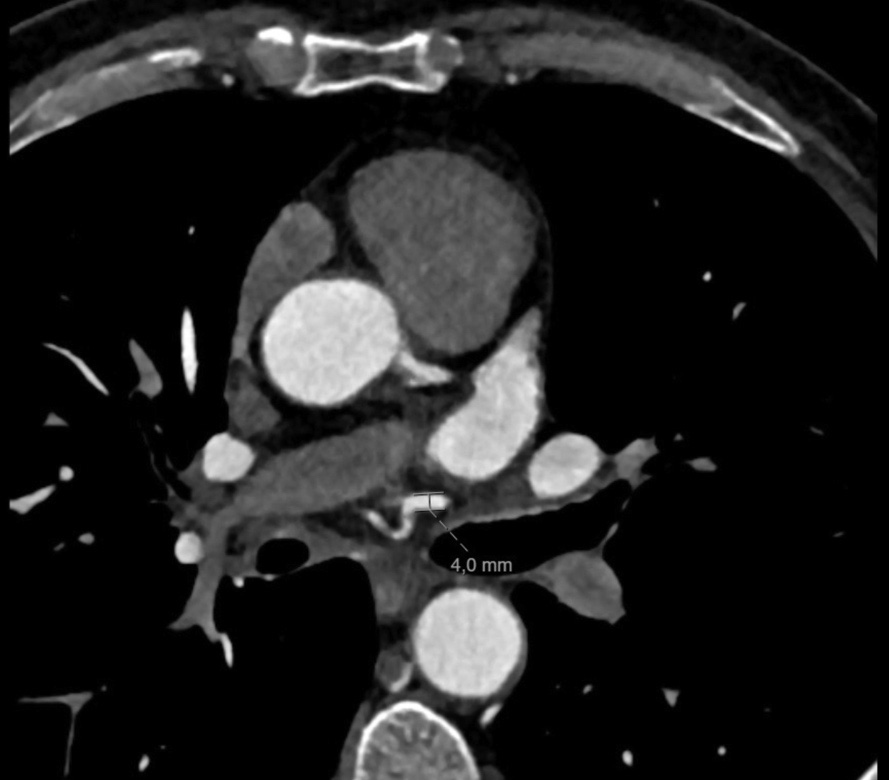
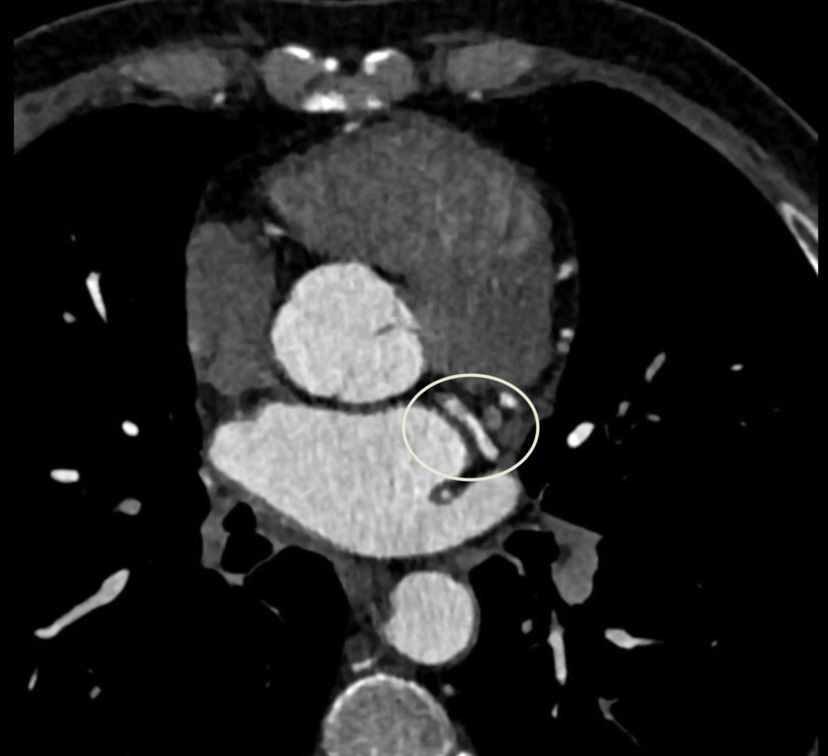
A 56-year-old man with significant smoking habits (44 pack years), dyslipidaemia, obesity and moderate alcohol consumption was admitted to the emergency service with exertional dyspnoea and angina. On admission he was polypnoeic and tachycardic, with intercostal retraction and oxygen desaturation – SpO2 88%. Pulmonary auscultation showed inspiratory crackles and sparse wheezing. Cardiac auscultation revealed two standard rhythmic sounds without murmurs. Electrocardiogram showed sinus rhythm 74/min, and inversion of T-wave in DII, DIII, aVF and V4 to V6. Blood tests revealed no elevation of myocardial necrosis markers but an elevation of natriuretic peptide (NT-proBNP 2913 pg/ml). Transthoracic echocardiography showed dilated cardiomyopathy with severe depression of left ventricular systolic function (25% Simpson’s ejection fraction) and marked global hypocontractility. A diagnostic cardiac catheterisation was proposed to clarify the ischaemic aetiology, which revealed severe coronary disease in two vessels: the anterior descending artery with a 90% lesion in the proximal segment, and the nondominant right coronary artery with an 80% lesion in the right ventricular branch. The same examination identified a large-calibre branch originating from the middle segment of the circumflex artery supplying the mediastinal structure. Because of these findings, a complementary study was performed with pulmonary computed tomography angiography and computed tomography coronarography, which showed an anomalous vessel originating from the circumflex artery (after the emergence of the first obtuse marginal branch), initially descending and later ascending, crossing the left pulmonary veins anteriorly. This vessel crosses the midline anteriorly of the right main bronchus (at this point it reaches a maximum calibre of 4 mm) and moves towards the right pulmonary hilum, irrigating the right bronchial tree, with findings suggestive of bronchiectasis without the presence of a mediastinal mass (Fig. 1,2). Given the potential consequences of coronary steal and the high risk of haemorrhage to the lung with severe haemoptysis if antiplatelet agents were used to treat his ischaemic disease, the patient was discussed in a multidisciplinary meeting (Internal Medicine, Cardiology, Cardiac Surgery and Radiology). Surgical intervention was proposed after confirmation of viability in the coronary territory of the descending anterior artery. Antiplatelet therapy was started and there was no evidence of pulmonary haemorrhage or other complications. The patient underwent surgery without intercurrences and was discharged on the seventh day.
DISCUSSION
Coronary artery fistulas are rare congenital or acquired defects in the coronary circulation that affect 0.1% to 0.2% of the population[1,3,5]. They are characterised by a connection between one or more coronary arteries and a cardiac chamber or a large vessel of the systemic or pulmonary circulation. Most coronary artery fistulae originate from the right coronary artery (40–60%) and mainly connect to the right atrium, right ventricle or pulmonary trunk[3]. Although it is a rare congenital or acquired entity, a CAF may present with different signs and symptoms depending on the number, size, location, haemodynamic profile and underlying comorbidities of the patient[1,3,4]. Clinical sequelae of CAFs may include dilatation of the heart chamber and dyspnoea, as well as ischaemia symptoms. Atrial fibrillation is perhaps the most common arrhythmia, although almost any arrhythmia can occur, especially when the atria and ventricles begin to dilate[5]. Multimodality imaging is essential for diagnosis and therapeutic decision-making. Coronary angiography and coronary computed tomography angiography (CTA) are considered highly reliable in the diagnosis of coronary artery fistulae. Reported outcomes from limited case series on transcatheter CAF closure demonstrate that this procedure is effective in most patients with suitable anatomy; however, it is not free of risks. The updated 2018 American College of Cardiology/American Heart Association guidelines recommend transcatheter closure or surgical closure for giant fistulas or when symptoms indicate obvious heart failure[4,5]. In our patient, given the coronary anatomy and large-calibre fistula, surgical intervention was suggested. However, the need for individual therapeutic decisions for each patient type should be made and discussed by a multidisciplinary team in centres with experience in both methods. This case highlights a rare entity that is usually detected during coronary angiography but, if symptomatic, may become relevant because of possible coronary steal syndrome.