ABSTRACT
Introduction: Organising pneumonia belongs to diffuse interstitial lung diseases; we distinguish the cryptogenic organising pneumonia, which is idiopathic, from the secondary organising pneumonia caused by drugs or a defined cause. Denosumab is a human monoclonal antibody, rarely inducing adverse pulmonary effects.
Case description: A 57-year-old female patient was admitted to our chest clinic for acute respiratory distress. She was treated with denosumab for severe osteoporosis. The patient described a dry cough and dyspnoea over the previous four months, increased after the last injection of denosumab. A high-resolution computed tomography scan showed bilateral basal parenchymal condensations. The aetiological investigation did not reveal any infectious or immunological origin. The favourable computed tomography imaging and clinical evolution after corticosteroid therapy led to the diagnosis of drug-induced organising pneumonia.
Conclusion: Denosumab could induce organising pneumonia. Therefore, clinicians should be aware of this pulmonary toxicity.
LEARNING POINTS
- To the best of our knowledge denosumab, a human monoclonal antibody, may rarely induce organising pneumonia.
- Despite this, we advocate that clinicians be aware that exposure to this drug can cause pulmonary toxicity.
- The taking of denosumab by our patient does not in any way prove the causal link.
KEYWORDS
Denosumab, secondary organising pneumonia, interstitial lung disease, osteoporosis
INTRODUCTION
Organising pneumonia (OP) is a clinical, radiological and histological entity belonging to the interstitial lung diseases (ILD). It is classified as cryptogenic OP (COP) if no cause is identified, or secondary OP (SOP) if caused by drugs, connectivitis, infectious damage or post radiation for example. Denosumab is a human monoclonal antibody widely used for treating osteoporosis and bone metastases, which may rarely cause adverse pulmonary effects[1]. However, since the receptor activator of nuclear factor kappa-B ligand (RANKL) and the receptor activator of nuclear factor kappa-B (RANK) are also expressed in the lung, denosumab might have adverse pulmonary effects, according to literature[2,3]. We report the case of a patient with severe osteoporosis under denosumab, who developed OP.
CASE DESCRIPTION
In December 2021, a 57-year-old non-smoking woman was admitted to our chest clinic for acute respiratory distress. Her past medical history was significant for hysterectomy and severe osteoporosis complicated by staggered vertebral fractures (T3, T4, T5, L1). She was treated with teriparatide over 22 weeks, then with denosumab from November 2020 (60 mg every six months). Her mother is hypertensive, her father died of prostate cancer and a sister died of pituitary cancer. She described a cough for the previous four months and increased dyspnoea following the last dose of denosumab (December 2021).
Physical examination revealed a polypnoeic patient with a respiratory rate of 27 breaths per minute; the oxygen saturation was 86% while the patient was breathing ambient air. She appeared to have dyspnoea mMRC grade 4 and a cough. Auscultation of the lungs revealed crackles in the left and right lower lung field; the remainder of the examination showed dorsal kyphosis.
METHODS AND PROCEDURES
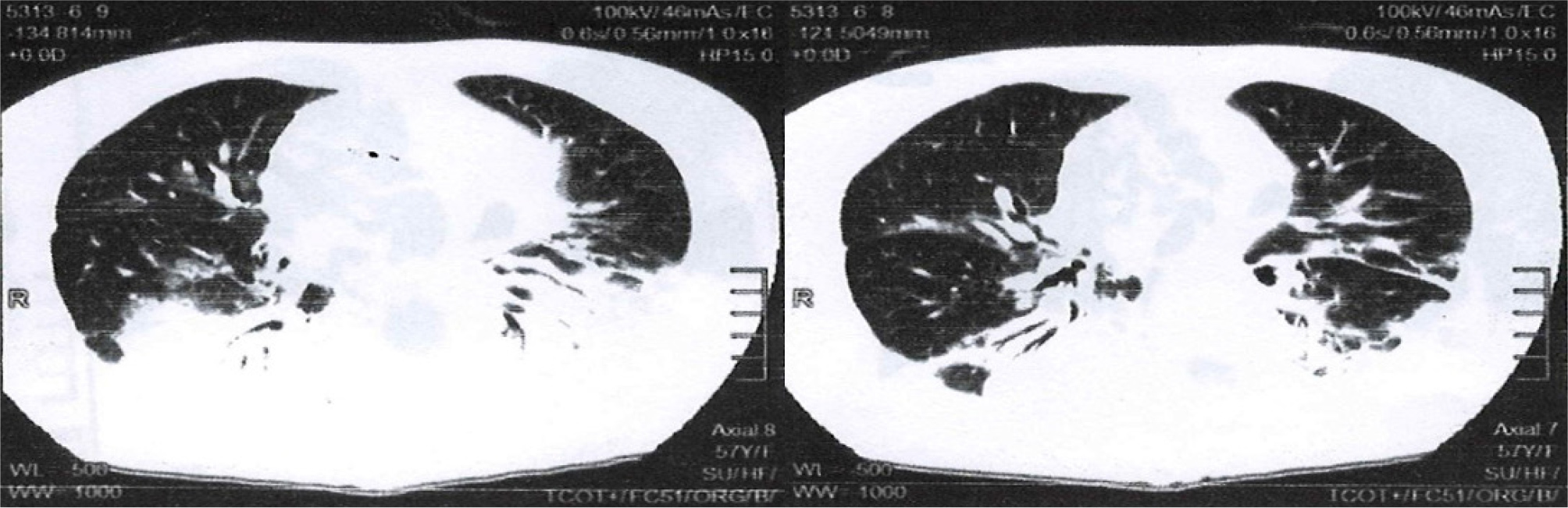
Blood levels showed a normochromic normocytic anaemia (haemoglobin at 9 g/dl), white blood cell count 22–38 × 109 and platelet count 90000/mm3. C-reactive protein, hepatic and renal tests, as well as the blood ionogram were normal. A SARS-CoV-2 antigen test and immunological assessment were negative. A chest computed tomography (CT) scan showed bilateral posterobasal consolidations with air bronchogram (Fig.1).
Figure 1. Chest CT, parenchymal window, axial section passing through the cardiac cavities showing bilateral postero-basal consolidations, under pleural, with an air bronchogram
The diagnosis of SOP was evoked because of a typical clinico-radiological picture, especially since the patient had taken her third dose of denosumab. Oxygen therapy at 4 l/min was initiated, as well as parenteral corticosteroid therapy based on methylprednisolone at 0.5 mg/kg/day for 10 days, followed by a per OS relay of prednisone at a rate of 40 mg/day, as an attack treatment, for 3 weeks. Calcium and potassium supplementation and gastric protection accompanied this corticosteroid therapy. Discontinuation of denosumab was proposed in communication with her treating rheumatologist.
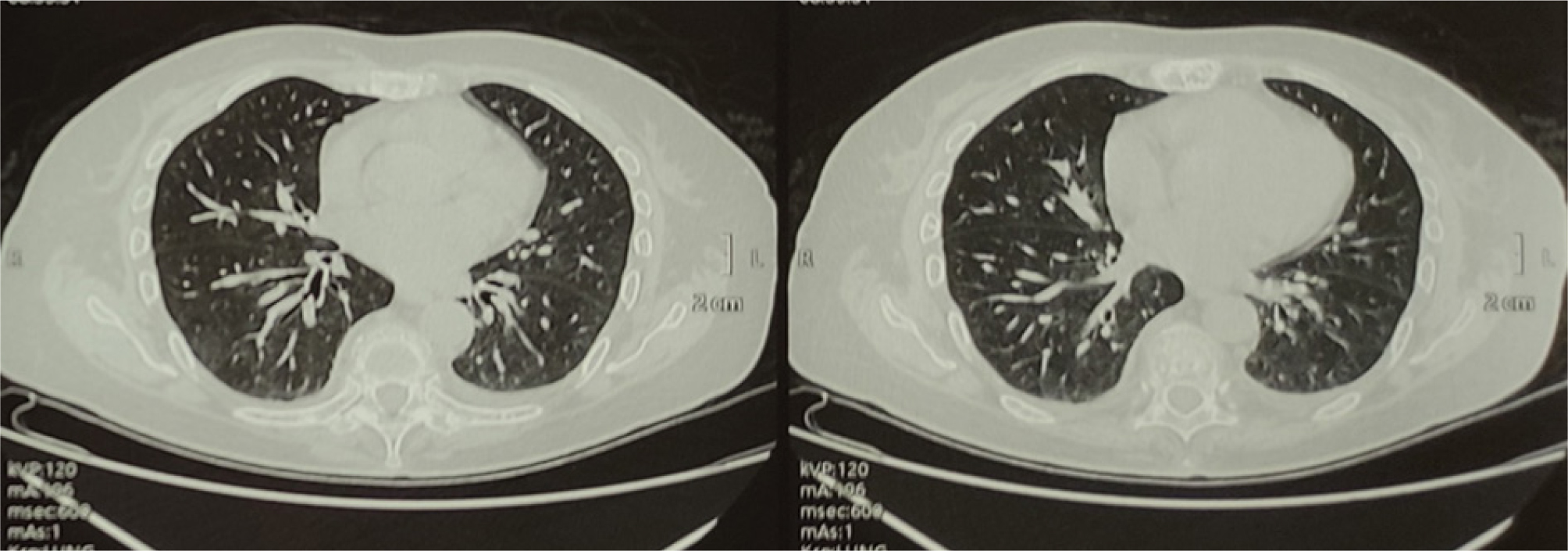
The clinical course was marked by an improvement in clinical signs and normalisation of oxygen saturation; the patient was discharged with gradual reduction of corticosteroid therapy over a period of 9 weeks. A chest CT scan, 3 months after the patient’s discharge, showed the disappearance of the pulmonary lesions (Fig. 2).
Figure 2. Chest CT, parenchymal window, axial section passing through the cardiac cavities showing the disappearance of the pulmonary lesions
DISCUSSION
Denosumab is a fully human monoclonal antibody (IgG2) that specifically binds to RANKL, inhibiting stimulation of RANK, for treatment of osteoporosis[4]. Its marketing authorisation application was approved by the United States Food and Drug Administration (FDA) in 2010 for the treatment of osteoporosis in postmenopausal women at high risk of fractures[5]. The marketing authorisation was subsequently obtained for other indications, in particular the treatment for bone loss in patients at high risk of fracture, during prostate cancer and breast cancer, as well as for glucocorticoid-induced osteoporosis[5].
Adverse effects of denosumab in the treatment of postmenopausal osteoporosis were assessed in a 3-year, multinational, randomised, double-blind, placebo-controlled study of 7,808 postmenopausal women, aged 60 to 91. More than 2% of women treated with denosumab experienced the adverse effects[6].
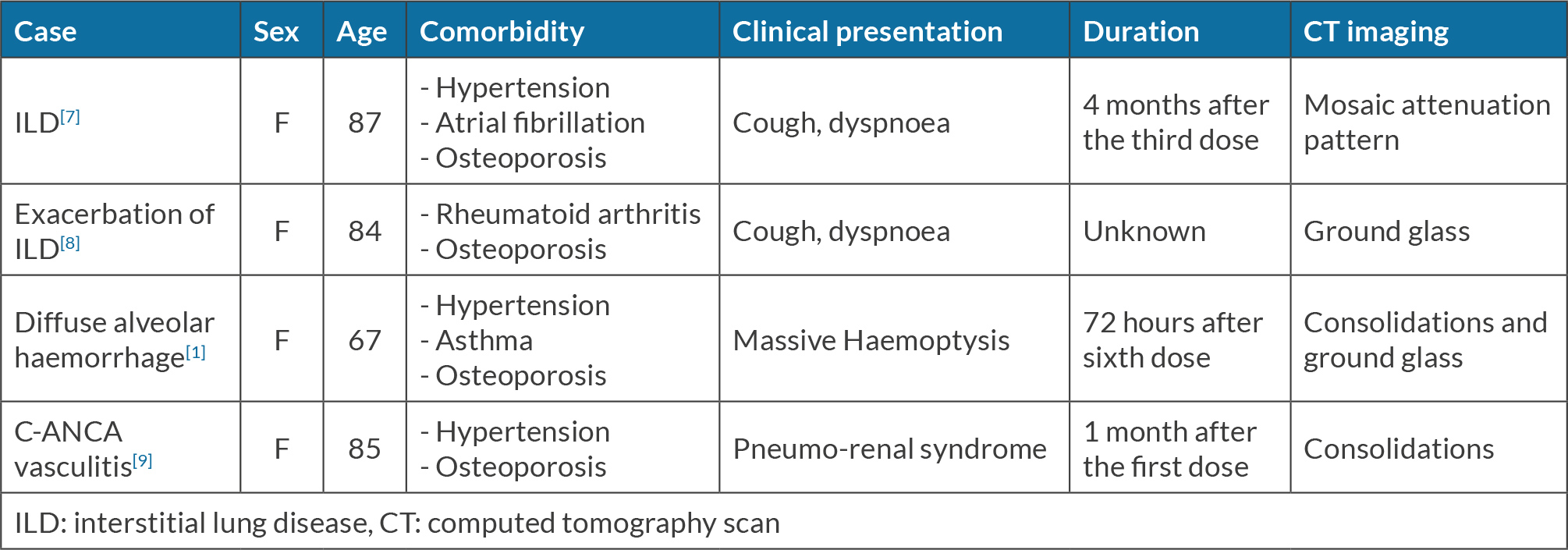
Denosumab is also responsible for different aspects of pulmonary toxicity reported in the literature; two cases of diffuse interstitial lung diseases[7,8], one case of c-ANCA vasculitis and one case of diffuse intra-alveolar haemorrhage due to p-ANCA have been described[9,1]. Descriptions of the clinical presentations for these reported cases are provided in Table 1.
According to a Japanese study reported in the Japanese Adverse Drug Event Report Database analysing the duration of onset of ILD induced by monoclonal antibodies, the median time to onset of ILD in 66 patients on denosumab was estimated at 64.5 days[10]. OP is a non-specific inflammatory response to injury to the lung parenchyma. It usually develops subacutely and manifests with a variety of symptoms, the most commonly reported being a cough and dyspnoea, with peripheral multifocal consolidations on chest imaging[11]. OP can be induced by drugs, of which 118 are reported on the Pneumotox site[12] including many monoclonal antibodies: nivolumab[13], tocilizumab[14] and rituximab[15], which can cause this lung disease. However, to the best of our knowledge, there have been no reported cases of SOP from denosumab.
In this observation the patient was already followed for postmenopausal osteoporosis and received three injections of 60 mg of denosumab subcutaneously, 6 months apart. She presented with acute dyspnoea 48 hours after the third injection.
Denosumab was the only medication she received, and the question was: is this clinical event an adverse drug reaction (ADR) related to denosumab? To answer this question we use the Naranjo score. This is widely used in clinical research and pharmacovigilance to determine the probability that an adverse effect is related to a specific drug, according to a pre-established questionnaire[16]. The score for this case report was calculated: (a) we cited two previous conclusive reports of this reaction (YES=+1), respectively, Ruis et al.[7] and Mori et al.[8]; (b) the clinical event appeared 48 hours after the third injection of denosumab (YES=+2); (c) the clinical event improved with denosumab withdrawal and corticosteroid therapy (YES=+1); (d) re-administration of denosumab was not done (=0), because of ethical aspects and the severity of the ADR; (e) there are no alternative causes (e.g. infection, immunological or post radiation origin) explaining this ADR (YES=+2). After evaluation of the remaining five questions, the total score was 6, therefore probable ADR=5–8.
On the other hand, a chest CT scan of this patient revealed bilateral consolidations with air bronchogram, subpleural, constituting a typical radiological pattern of OP[17]. The clinical evolution, after discontinuation of denosumab and under corticosteroid therapy, was favourable, with disappearance of the pulmonary lesions three months later. It should be noted in our observation the absence of histological proof of OP, and the non-confirmation of the causal link by reintroduction of the drug.
In summary, the history of denosumab exposure, the clinical event, the absence of alternative causes, the probable ADR Naranjo score, the favourable clinical and radiological outcome after the discontinuation of denosumab and the effect of corticosteroid therapy reinforce the likely causal relationship between denosumab and the pulmonary toxicity. Therefore, the diagnostic hypothesis of secondary organising pneumonia characterised by a typical clinico-radiological picture and probably related to denosumab should be possible.
CONCLUSION
Denosumab seems to be responsible for organising pneumonia, an unusual clinical situation of pulmonary toxicity. It should be emphasised in our clinical case that the taking of denosumab by our patient does not in any way prove the causal link. In addition, practitioners should consider potential pulmonary side effects when prescribing denosumab, and therefore monitor patients receiving this treatment.