ABSTRACT
Acute pulmonary thromboembolism (PTE) is considered the third most frequent acute cardiovascular syndrome behind myocardial infarction and stroke, with annual incidence rates ranging from 39 to 115 per 100,000 people and ranking high among the causes of cardiovascular mortality. High-risk PTE is characterised by haemodynamic instability and encompasses clinical manifestations such as cardiac arrest, obstructive shock and persistent hypotension. The European Society of Cardiology (ESC) recommends a reperfusion strategy with systemic thrombolytic therapy for high-risk PTE under class I, level B if there are no contraindications. Overall, unsuccessful thrombolytic therapy and recurrent PTE have been reported in 8% of patients with high-risk PTE. The guidelines recommend surgical pulmonary embolectomy if thrombolysis is contraindicated or has failed. The position of repeated thrombolytic therapy as a treatment option in patients with recurrent high-risk PTE, especially in situations with a lack of surgical expertise or resources, was not mentioned in the guidelines. We report the case of a patient who suffered a recurrent high-risk PTE and was treated with repeated thrombolytic therapy that was effective and resulted in excellent outcomes.
LEARNING POINTS
- Acute pulmonary thromboembolism (PTE) is a serious medical condition and widespread disease with well-recognised morbidity and mortality.
- Systemic thrombolytic therapy should be the first choice in patients with high-risk PTE without contraindication.
- Repeated thrombolytic therapy in recurrent high-risk PTE might be effective in patients with low risk of bleeding as an alternative to surgical embolectomy or catheter-directed therapy.
KEYWORDS
High-risk pulmonary thromboembolism, alteplase, systemic thrombolysis
INTRODUCTION
Acute pulmonary thromboembolism (PTE) is a serious medical condition and widespread disease with well-recognised morbidity and mortality. Both circulation and gas exchange are affected in acute PTE due to acute right ventricular (RV) pressure overload because of clot burden and increased pulmonary vascular resistance that lead to RV failure; this is considered the primary cause of death in severe pulmonary embolism. High-risk PTE is a life-threatening situation that needs immediate diagnostic and therapeutic strategy upon suspicion and confirmation, respectively. Thrombolytic therapy is usually reserved for patients with high-risk PTE in the absence of contraindication. The role of repeated use of thrombolytic therapy in the management of patients with recurrent PTE is unclear. Our case demonstrated a patient who developed a high-risk PTE treated initially with reteplase. After a few days, he had a recurrent high-risk PTE managed with the administration of alteplase, taking into consideration his low bleeding risk profile. His clinical condition significantly improved, and he was discharged home a few days later. There is little published evidence on repeated thrombolytic therapy for recurrent high or intermediate-risk PTE. A few case reports in the literature demonstrated the use of repeated thrombolytic therapy in recurrent pulmonary embolism.
CASE DESCRIPTION
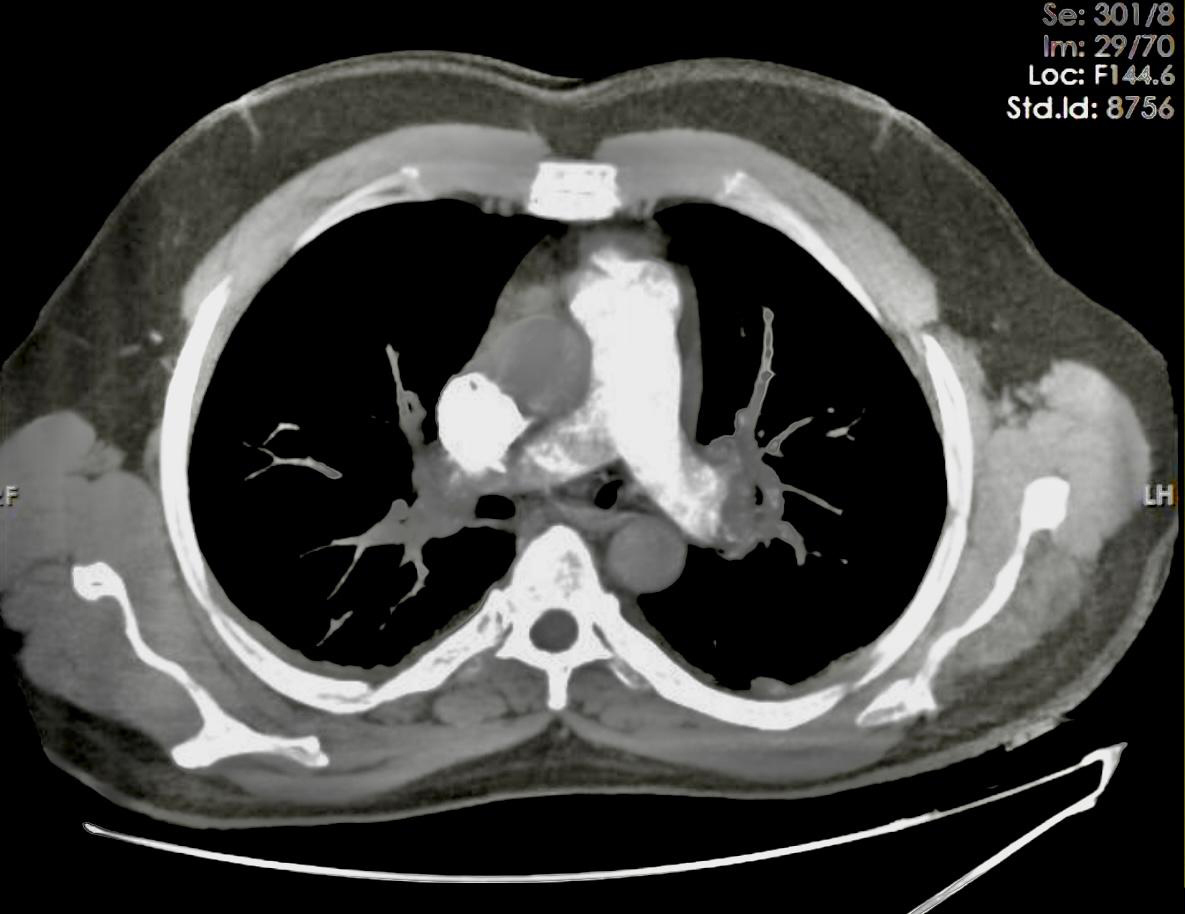
A 45-year-old man presented to our emergency department complaining of significant shortness of breath for the previous few hours. He gave a history of regular use of dexamethasone tablets for the past few years to gain weight and also admitted to being addicted to opium. The patient is a heavy smoker and denied significant allergies. On admission, he was agitated with oxygen saturation of 77% on room air and 90% with oxygen supplement. His blood pressure was 90/50 mmHg and his heart rate was 120 beats per minute. Heart auscultation showed only tachycardia, while breath sounds were normal on chest examination. His electrocardiogram revealed sinus tachycardia. Bedside echocardiography showed severe RV enlargement and dysfunction, positive McConnell’s sign and elevated systolic pulmonary artery pressure of 55 mmHg. A computed tomography pulmonary angiogram (CTPA) revealed multiple filling defects suggestive of PTE (Fig. 1). Laboratory tests showed elevated cardiac troponin and brain natriuretic peptide. The patient made a similar presentation at another cardiac centre 10 days previously, where he was diagnosed with high-risk PTE and treated with thrombolytic therapy, particularly reteplase (alteplase was not available in that centre). The patient showed clinical and echocardiographic improvement and was discharged home on oral anticoagulant. Unfortunately, the patient was not compliant with his medication and presented to our centre with a similar picture five days after his discharge. His condition was suggestive of recurrent high-risk PTE based on his clinical picture, with hypotension in addition to echocardiographic and CTPA findings on presentation.
Figure 1. Multiple filling defects in both pulmonary arteries are seen in the computed tomography and pulmonary angiogram
Although reteplase is considered a thrombolytic agent, this was not recommended in the ESC Guidelines. Instead, alteplase is the recommended agent for thrombolytic therapy. Since our patient presented with a recurrent high-risk pulmonary embolism and considering his low bleeding risk profile, the decision was made to repeat the thrombolytic therapy with alteplase (100 mg intravenous infusion over 2 hours). The patient showed marked improvement clinically with normalisation of blood pressure, and follow-up echocardiography within 36 hours after thrombolytic administration revealed recovery of RV function and reduction of pulmonary artery pressure. He was discharged on oral anticoagulation with a recommendation to stop the use of dexamethasone tablets, and to stop smoking and consumption of opium. The patient was seen in the outpatient clinic after two weeks in good health with normalisation of the echocardiographic picture.
Our case demonstrated a partial response to initial thrombolytic therapy and a recurrent episode of PTE in a young patient with low bleeding risk. Although guidelines recommend surgical pulmonary embolectomy if thrombolysis fails, the role of repeated thrombolytic therapy as a treatment option in patients with a recurrent high-risk PTE is not clear. This is especially the case in situations with a lack of surgical expertise or resources on site for low bleeding risk profiles. Reteplase is not recommended by the ESC Guidelines to be used as a thrombolytic agent for high-risk PTE. Given recurrent high-risk PTE after initial use of reteplase with low bleeding risk, repeated thrombolytic therapy with alteplase was given instead of surgical pulmonary embolectomy, with a dramatic response and good outcome. These cases showed that repeated thrombolytic therapy in recurrent high-risk PTE might be effective in patients with a low risk of bleeding as an alternative option to surgical embolectomy or catheter-directed therapy.
DISCUSSION
After myocardial infarction and stroke, venous thromboembolism (VTE) clinically manifested as deep venous thrombosis or pulmonary embolism scored the third most encountered acute cardiovascular event in daily practice[1]. Acute PTE is a serious medical condition and widespread disease with well-recognised morbidity and mortality. The epidemiological trials of pulmonary embolism revealed an annual incidence rate between 39 and 115 per 100,000 people, whereas the longitudinal studies emphasised the growing tendency in the annual incidence of pulmonary embolism[2]. The combination and interaction between long-standing risk factors that usually relate to the patient and temporary risk factors that are usually related to the setting may result in venous thromboembolism. The balance between these factors plays a major role in decision-making on chronic anticoagulation. A strong risk factor for VTE is orthopaedic pathology and procedures (lower-limb fractures, spinal cord injury and joint replacements), surgery and major trauma[3]. A well-recognised risk factor for VTE is cancer where different types of malignancy carry a variable risk of VTE, with the highest risk considered with haematological malignancies, pancreatic, lung, gastric and brain cancer[4,5]. The main cause of deterioration in acute pulmonary embolism that may lead to death is acute pressure overload with subsequent right ventricular (RV) failure that interferes with both circulation and gas exchange. The sudden increase in pulmonary vascular resistance during acute pulmonary embolism is explained by an anatomical obstruction in addition to vasoconstriction mediated by hypoxia and released mediators such as thromboxane A2 and serotonin with subsequent reduction of arterial compliance[6]. The thin-walled RV is unable to face such a sudden increase in afterload. The contractile function of RV will be exhausted soon since immediate adaptation is quite limited. This will lead to decreased filling of the left ventricle and cardiac output, resulting in haemodynamic deterioration and circulatory shock[7]. The elevation of biomarker levels in circulation in the acute phase of pulmonary embolism showed a significant association with an early adverse outcome. It demonstrates the presence of RV ischaemia that progressively increases due to impairment of the coronary driving pressure because of systemic hypotension. The determinant of clinical severity and outcome in acute pulmonary embolism will depend on how far and rapid the pathophysiological mechanism of acute RV failure syndrome with associated systemic congestion will develop. Based on these pathological processes, it is crucial to determine the risk stratification of acute PTE to select the appropriate therapeutic management approach. PTE is commonly classified as high-risk, intermediate-risk or low-risk PTE. High-risk PTE is characterised by haemodynamic instability and encompasses clinical manifestations such as cardiac arrest, obstructive shock and persistent hypotension. In the absence of shock, suspected or confirmed PTE with RV dysfunction is classified as an intermediate-risk PTE. ESC recommends a reperfusion strategy with systemic thrombolytic therapy for high-risk PTE under class I, level B if there are no contraindications[8]. Although thrombolysis in patients with symptoms can still be useful for 6–14 days after the onset of symptoms, the biggest benefit of treatment is observed when thrombolysis is initiated within 48 hours of the onset of symptoms[9]. Compared with unfractionated heparin (UFH) alone, thrombolysis leads to faster recovery in pulmonary obstruction, pulmonary artery pressure and pulmonary vascular resistance, and these improvements are usually associated with reduction in RV dilation via echocardiography[10]. The high-risk patients included in different trials and treated with thrombolysis were reviewed in a meta-analysis of thrombolysis trials and a significant decrease was observed in the combined outcomes of mortality and recurrent event at the expense of a 9.9% rate of severe bleeding and a 1.7% rate of intracranial haemorrhage[8]. Unfortunately, 8% of patients with high-risk PTE showed a failed response to thrombolysis that can be detected after 36 hours by persistent clinical instability and unchanged RV dysfunction on echocardiography[11]. In 2006, Meneveau et al. reviewed patients with acute massive PE who failed thrombolytic treatment and concluded that surgical embolectomy provides a better course in the hospital than a repeated thrombolytic treatment[11]. Azari et al. reported a prospective study of 30 patients who underwent emergency embolectomy with indications according to the last American Heart Association Guidelines; 26.6% of patients failed to respond to initial thrombolysis. The researchers concluded that short- and long-term outcomes of early open embolectomy seemed to be satisfactory in high-risk patients presenting with high clot burden in central pulmonary arteries[12]. ESC recommended surgical pulmonary embolectomy for patients with high-risk PTE, where thrombolysis is contraindicated or has failed[13]. Azari et al. compared surgical embolectomy and thrombolytic therapy in patients suffering from high-risk PTE and concluded that early surgical treatment was associated with fewer complications than thrombolytic therapy[14]. Reperfusion therapy with thrombolysis targets thrombus dissolution with immediate restoration of vascular patency. This has the potential benefits of immediate relief of symptoms with prevention of clinical deterioration and long-term complications, such as chronic thromboembolic pulmonary hypertension (CTEPH), considered an important cause of severe pulmonary hypertension resulting in significant morbidity and mortality. The gold standard and effective treatment for CTEPH is pulmonary endarterectomy[15]. The latest ESC Guidelines for pulmonary embolism mention the approved regimens and doses of thrombolytic agents, including recombinant tissue plasminogen activator (alteplase), streptokinase and urokinase. In the same guidelines, it was mentioned that the accelerated intravenous administration of recombinant tissue-type plasminogen activator (alteplase of 100 mg over 2 hours) is preferable to prolonged infusions of first-generation thrombolytic agents (streptokinase and urokinase). Tissue plasminogen activators (tPA) are protease enzymes that cleave peptide bonds in proteins. They are considered the essential components of the dissolution of blood clots by catalysing the conversion of plasminogen to plasmin that has the main role in dissolving blood clots. Alteplase, reteplase and tenecteplase are the available examples of tPA; however, the only tPA approved for use in high-risk PTE is alteplase[16]. The increased risk of major haemorrhage must be taken into consideration. Registry data and data from existing randomised controlled studies suggest that in the specific setting of PE, thrombolysis is associated with major bleeding rates of 10%–20%[17,18].
As reported in the guidelines, 8% of patients with high-risk PTE had reported unsuccessful thrombolytic therapy and recurrent PTE. The role of repeated thrombolytic therapy as a treatment option in patients with recurrent high-risk PTE, especially in situations with a lack of surgical expertise or resources and low risk of bleeding, was not mentioned. In the literature, a few case reports demonstrated the use of repeated thrombolytic therapy in recurrent pulmonary embolism. Argüder et al. reported two cases with intermediate- to high-risk PTE treated initially with thrombolytic therapy with good recovery; however, after a few days, both patients had evidence of recurrent high-risk PTE and were treated with repeated doses of alteplase. The first patient was treated successfully, while the second died due to haemodynamic collapse and gastrointestinal bleeding[19]. Another case series of two patients reported by Poor et al. demonstrated successful treatment of refractory massive pulmonary embolism with repeated administration of systemic thrombolysis[20]. Both patients were initially treated with thrombolytic therapy due to massive pulmonary embolism and obstructive shock; however, they remained in obstructive shock requiring significant inotropic and vasopressor support. Both patients were deemed poor candidates for embolectomy. Repeated thrombolysis with alteplase was given for both patients. The first patient received repeated doses reaching an accumulative dose of 200 mg of alteplase over 15 hours. The second patient received an accumulative dose of 250 mg of alteplase over 36 hours. The resolution of shock within 24 hours of repeated administration of alteplase was documented; also, significant drops in haemoglobin were experienced, which were supported with transfusions. One patient was successfully weaned from the ventilator and discharged home while the other had a complicated course of invasive candidiasis with septic shock, and expired after one week.
Our patient demonstrated a high-risk PTE that was initially treated with the non-approved thrombolytic agent for pulmonary embolism and had a recurrent episode of pulmonary embolism. His bleeding risk profile was low, and his response to the repeated thrombolytic therapy with alteplase was clearly good; he was discharged home in good condition.
CONCLUSION
Thrombolysis should be the first choice in patients with high-risk PTE in the absence of contraindication. Repeated thrombolytic therapy in recurrent high-risk PTE might be effective in patients with a low risk of bleeding as an alternative to surgical embolectomy or catheter-directed therapy.