ABSTRACT
Spontaneous coronary artery dissection (SCAD) is an epicardial coronary artery dissection not associated with atherosclerosis or trauma, and is not iatrogenic. The pathogenesis of SCAD is not fully understood, and the association of coronary artery anomalies and SCAD is not known. We present a case of a 64-year-old woman presenting with non-ST-elevation myocardial infarction (NSTEMI) due to have multivessel SCAD in the setting of anomalous origin of the coronary arteries with all three coronary arteries – right coronary artery (RCA), left anterior descending artery (LAD), and left circumflex artery (LCx) originating from the right sinus of Valsalva.
LEARNING POINTS
- Spontaneous coronary artery dissection (SCAD) and coronary artery anomaly can arise where all three arteries originate from the right coronary cusp;
- There are possible associations between SCAD and coronary artery anomalies;
- Treatment and management of multivessel SCAD with a coronary artery anomaly where all three arteries arise from the right sinus of Valsalva.
KEYWORDS
Dissection, anomaly, myocardial infarction
INTRODUCTION
Spontaneous coronary artery dissection (SCAD) is prevalent in middle-aged women, with a mean age between 44 and 53 years[1,2]. It is hypothesised to be multifactorial, often compounded by environmental factors and stressors[1]. The underlying mechanism is not completely understood, with two possible theories proposed. The first theory proposes an intimal tear as the primary event enabling blood to enter and generate a false lumen, leading to an intramural haematoma known as the inside-out hypothesis[3]. The second theory proposes a primary disruption of the vasa vasorum, creating haemorrhage into the vessel, and developing an intramural haematoma without an intimal tear[3]. The increased pressure within the vessel wall causes a rupture into the true lumen resulting in an intimal rupture, and is known as the outside-in hypothesis[3]. SCAD is rarely reported in the presence of coronary artery anomalies. There are limited case reports and studies that report SCAD with a coronary artery anomaly; however, due to its rare nature, there is yet to be found evidence to show the association between these uncommon findings.
CASE PRESENTATION
A 64-year-old woman presented with migraine and chest discomfort. She reported a recent increase of stressors in her life. On examination, vital signs showed elevated pressure of 161/101 mmHg, and a heart rate of 55 bpm, without murmurs. She was incidentally COVID-19-positive without symptoms.
Medical history was significant for controlled hypertensions and migraines, with rescue sumatriptan as needed. She had no prior cardiac history or risk factors of tobacco use or alcohol consumption.
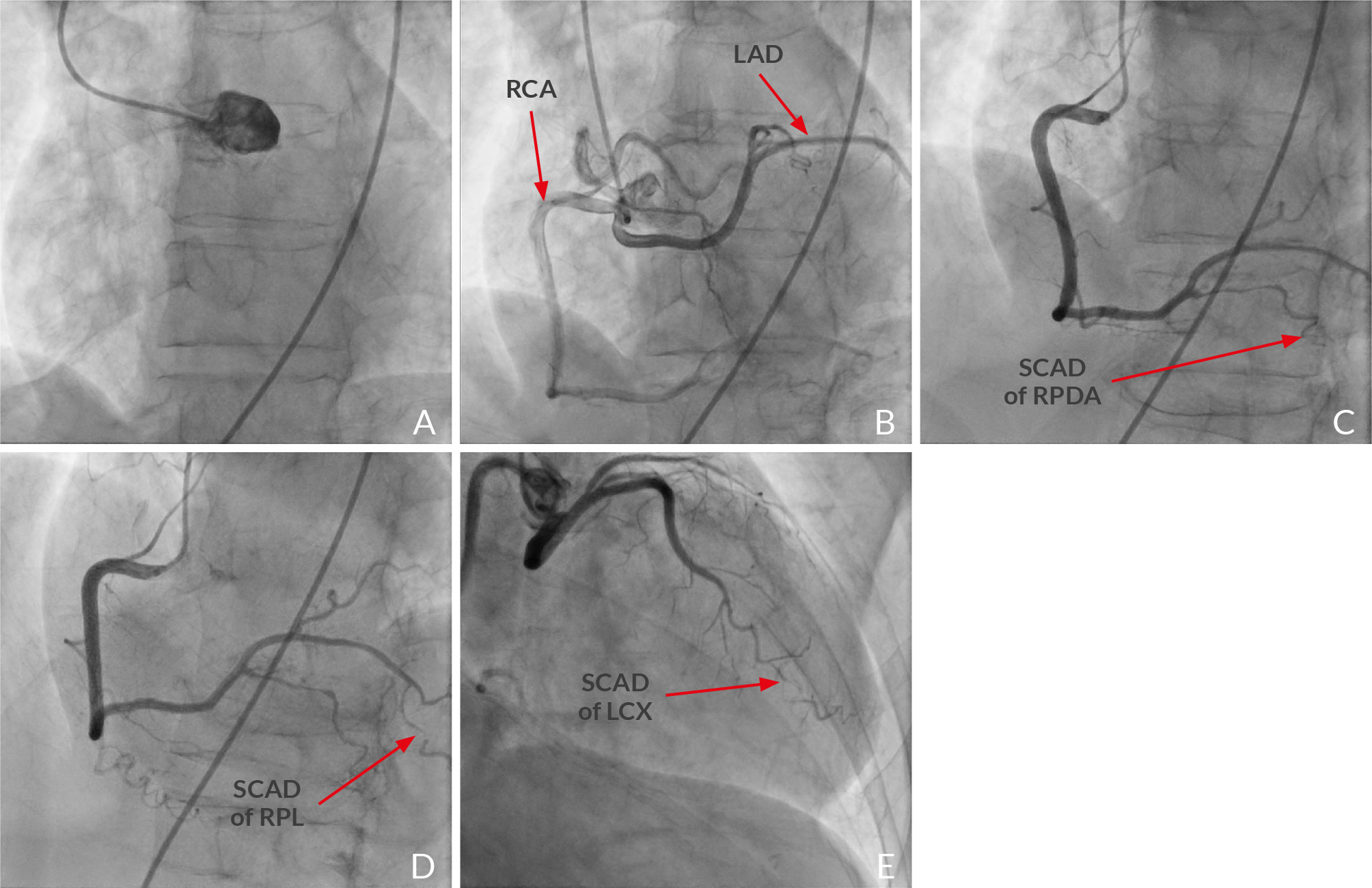
An electrocardiogram showed sinus bradycardia without ischaemic ST-T changes. On lab work, the patient had a repeated troponin test that was initially elevated at 351 ng/l (normal ≤ 10 ng/l). At two hours troponin was elevated at 393 ng/l, and 710 ng/l at the 6-hour mark indicating an NSTEMI. The echocardiogram showed preserved ejection fraction (EF) of 63% with new regional wall motion abnormalities of the lateral and apical walls. A coronary angiogram (Fig. 1, video) revealed an anomalous origin of the LAD and LCx arising from the right coronary cusp (RCC) (Fig. 1B). Additionally, there was multivessel type 2 SCAD of the right posterior descending coronary artery, distal right posterolateral branches and distal LCx arteries (Fig.1C, 1D and 1E).
Several considerations were taken into account after the diagnosis of multivessel SCAD with anomalous coronary artery origins from the RCC. First, the patient was haemodynamically and electrically stable with preserved EF. Additionally, the SCAD was located distally and the anomalous origin would make percutaneous intervention more difficult, so conservative management was preferred. The patient was also started on carvedilol 3.125 mg BID, 40 mg atorvastatin, and dual antiplatelet therapy of clopidogrel and aspirin. Sumatriptan was discontinued.
Figure 1. Coronary angiography of a coronary artery anomaly with multivessel spontaneous coronary artery dissection
A) Angiographic image of the left cusp that shows the absents of a LCA originating from the left sinus of Valsalva; B) Image of the right cusp showing origins of a RCA and LAD. A Tortuous LAD with no indication of SCAD; C) SCAD of RPDA; D) SCAD of the distal RPL; E) Image showing origins of a LCX from the right cusp with a retro-aortic course and SCAD of distal LCX
Video.
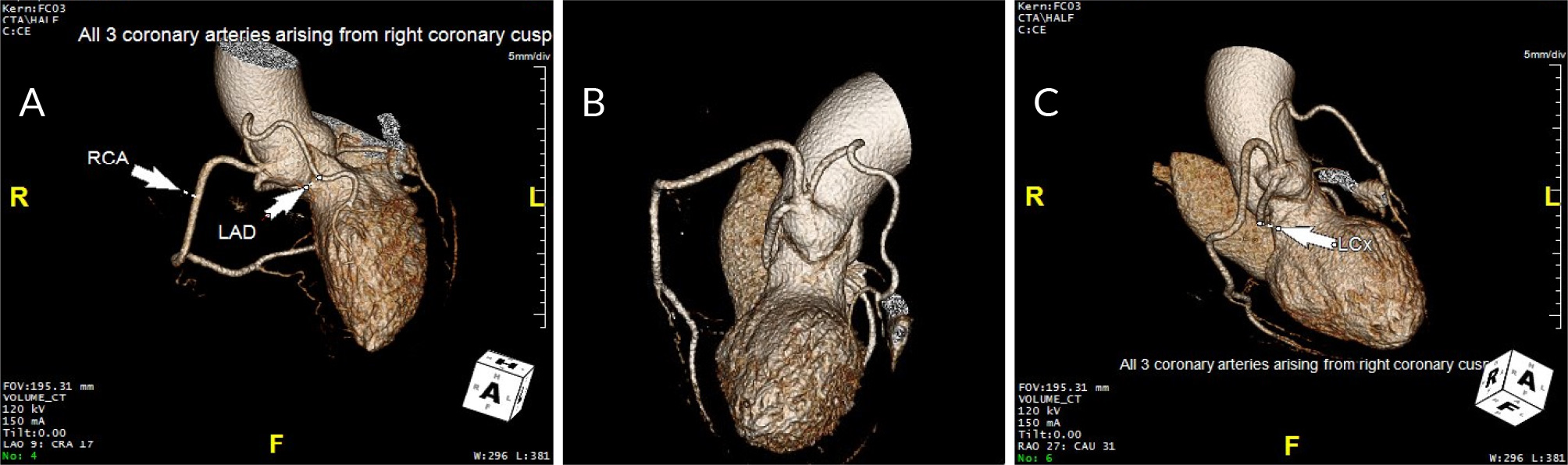
Figure 1. Computed tomography angiography of a coronary artery anomaly
A, B) CTA showing the RCA, LAD, and LCX originating from the right cusp; C) Imaging showing the LCX having a retro-aortic course
DISCUSSION
SCAD is an acute coronary syndrome that is often missed and unreported due to a low index of suspicion and unfamiliarity with SCAD’s angiographic variants[8]. The most common coronary artery to dissect is the left anterior descending artery[1,2,8]. The LAD is affected in 75%, RCA in 20%, LCx in 4% and the LMCA in less than 1% of cases[4]. This case represented a rare occurrence of SCAD with multivessel involvement in which the LAD was spared. Conservative management is recommended if the patient is stable and does not have proximal or multivessel dissection[1]. Despite the multivessel dissection in this patient, conservative management was decided upon due to the coronary anomalies and the patient being haemodynamically stable. Studies have also shown that SCAD arteries heal spontaneously, and that percutaneous coronary intervention carries higher rates of complication and procedural failure, and does not protect against recurrent SCAD events[8]. The recurrence of SCAD is common. A 10-year recurrence rate for dissection is 29%, with a median time for a second episode is 3 years[8]. With the chance of reoccurrence, it is essential for clinicians to follow patients closely who have had SCAD in the past.
Many factors can contribute to the development of SCAD. The patient admitted to having stress in her life and has a history of migraine on sumatriptan. Studies have shown that 37%–46% of SCAD patients in a cohort study had a history of migraines[9]. Perez et al. even showed additional evidence that triptans, such as sumatriptan, may increase artery dissection risk in patients with migraines[10]. Can a coronary anomaly also be a contributing factor? A coronary artery anomaly where all coronary arteries originate from the right sinus of Valsalva is extremely rare, with a reported incidence of 0.008% in angiographic studies[11]. Most coronary artery anomalies are benign. However, specific anomalies can create significant clinical cases and be predisposing factors for myocardial ischaemia. The origin of a LAD and LCx from the opposite cusp can be a potential serious coronary anomaly that can lead to myocardial infarction and sudden death[12,13]. A case series of anomalous left circumflex arteries showed that plaque rupture was more common in anomalous LCx, compared with the non-anomalous LCx group, due to their tortuous course and the shear stress alteration within the vessel[14]. The retroaortic LCx anomaly is selectively predisposed to atherosclerotic disease[14]. This patient had an LCx retroaortic course that was found to have non-atherosclerotic SCAD of the distal LCx artery. An angiogram also showed SCAD of the right posterior descending coronary artery and distal right branches of the RCA, indicating multivessel SCAD. Coronary artery anomalies are not a known risk factor for SCAD, therefore we wanted to highlight a rare combination of SCAD and single sinus origin of all coronary arteries.
Two weeks after discharge the patient was evaluated at her cardiology clinic, and no longer had any chest pain. Computed tomography angiography (CTA) was obtained 2 months later due to abnormal creatinine levels. CTA showed no significant stenosis and a coronary artery calcium Agatston score of 0 with near resolution of SCAD (Fig. 2).
CONCLUSION
This is a rare case of SCAD where all three coronary arteries arise from the right cusp. This can have significant implications and adds complexity to interventional therapies if these are required.