ABSTRACT
Cardiac conduction disorder may have a wide range of aetiology and can manifest with symptomatic bradycardia and syncope. Celiac disease is a malabsorptive long-term autoimmune disorder where the small intestine is the primarily affected organ due to gluten intolerance in genetically predisposed individuals. The associations between celiac disease and cardiac pathology are uncommon. We report a case of a 50-year-old woman with a known case of celiac disease who presented with a symptomatic cardiac conduction abnormality that improved with a gluten-free diet.
LEARNING POINTS
- Celiac disease is a malabsorptive long-term autoimmune disorder where the small intestine is the primarily affected organ due to gluten intolerance and affects 1% of the general population.
- Cardiovascular pathology, including dilated cardiomyopathy, myocarditis, arrhythmias, and premature atherosclerosis, was found to be more prevalent in patients with celiac disease than in others without celiac disease.
- The association of celiac disease with isolated advanced atrioventricular conduction abnormality is rare and a gluten-free diet may help improve the conduction abnormality.
KEYWORDS
Complete heart block, celiac disease, malabsorption
INTRUDUCTION
Cardiovascular pathology, including cardiomyopathy, myocarditis, arrhythmias, and premature atherosclerosis, was found in the literature to be more common in individuals with celiac disease than in those without the disease. The cardiac conduction abnormality as a possible association with celiac disease was mentioned only in a few case reports. However, in these cases, the authors attributed the infranodal heart block to other associated conditions like idiopathic pulmonary hemosiderosis. In our case, the patient had a documented history of celiac disease and the workup showed no other contributing conditions that can explain the cardiac conduction abnormality, which may raise the possible association of celiac disease and conduction abnormality, especially with significant improvement of the cardiac conduction system after a strict gluten-free diet.
CASE DESCRIPTION
A 50-year-old woman with no previous medical problems apart from celiac disease was diagnosed 10 years ago. At that time, she was investigated for general weakness and anaemia and found to have high titer of specific antibodies and celiac disease found on the small intestine biopsy. She was not strictly compliant with a gluten-free diet and was asymptomatic for the past five years. She is a nonsmoker and denied any medication, recreational drugs, or alcohol intake, and had no history of allergies. Her family history was unremarkable for celiac disease or other autoimmune conditions. She presented to the emergency department after she experienced an episode of loss of consciousness while in her home. She reported episodes of dizziness and near syncope that improved spontaneously during the last week. She also reported no infectious symptoms and had no chest pain, palpitations, dyspnoea, orthopnoea, or paroxysmal nocturnal dyspnoea. She denied gastrointestinal symptoms. On arrival, she was conscious but feeling dizzy.
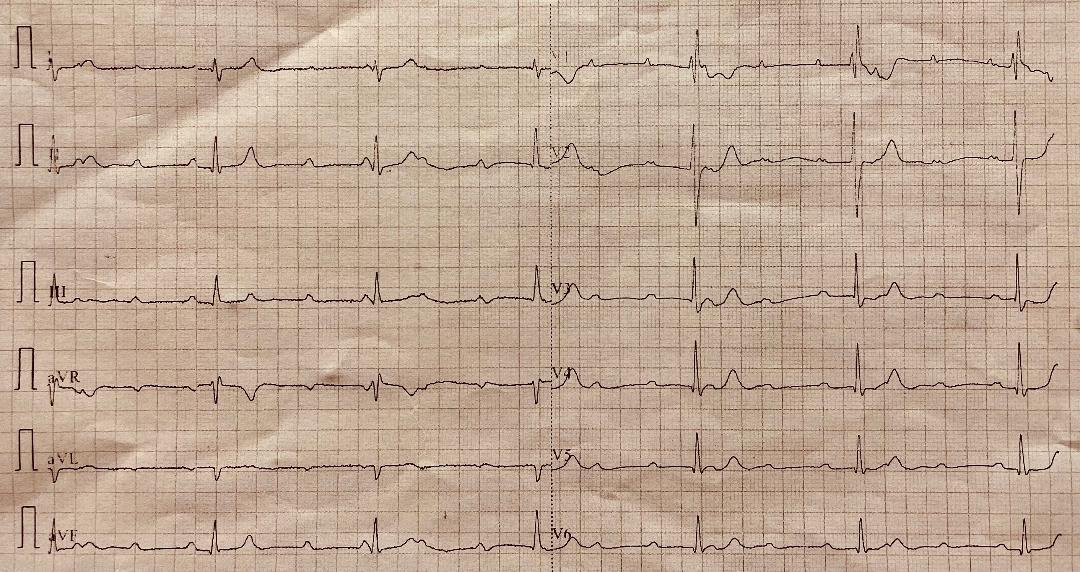
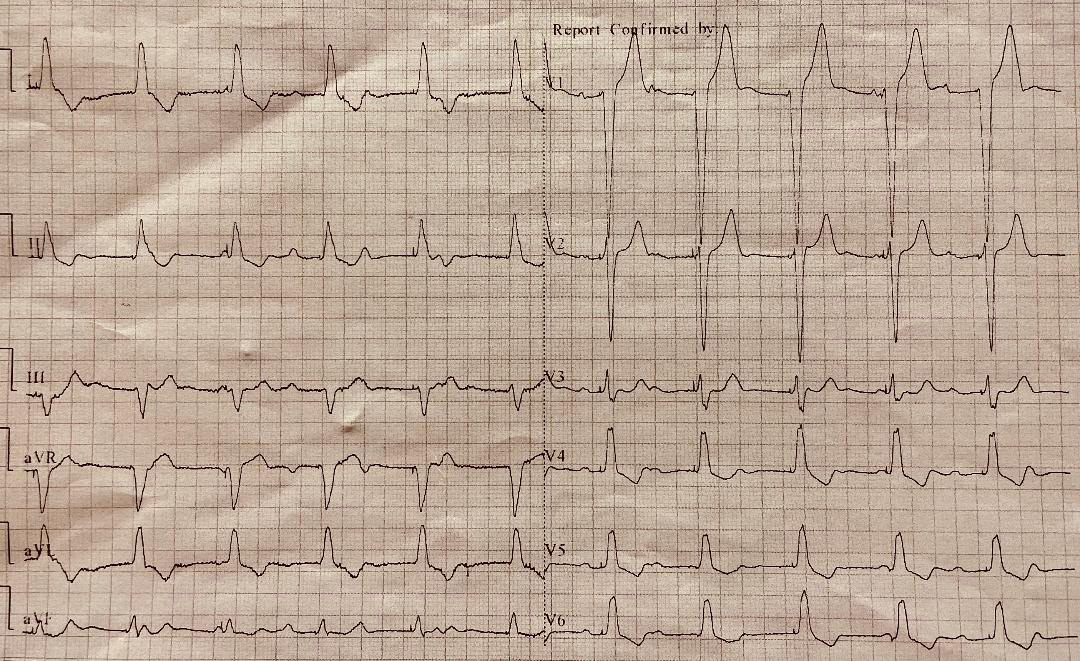
She was underweight, with a body weight index of 17. Her vital signs showed a blood pressure of 130/80, a heart rate of 38 beats per minute (B/min), and oxygen saturation of 98% on room air. Besides being underweight and bradycardic, her physical examination was within normal range. The electrocardiography (ECG) showed a complete hearty block with a ventricular escape rhythm of 38 B/min (Fig. 1). She was given atropine intravenously in repeated doses with temporary improvement in heart rate and her symptoms. She was shifted to the intensive care area and started on dopamine infusion, which improved her heart rate to 70 B/min, and she was asymptomatic. The transthoracic echocardiography and computed tomography coronary angiography were normal. Her laboratory investigation showed only microcytic hypochromic anaemia with normal white cell count and platelet count. An iron study showed low iron and ferritin levels with iron deficiency anaemia, which excluded the diagnosis of hemochromatosis. Her electrolytes and renal, liver, and thyroid functions were normal, as well as troponin and C-reactive protein. Autoimmune screen (including anti-extractable nuclear antigen, rheumatoid factor, and anti-DNA antibody) was negative apart from high serum anti-tissue transglutaminase antibodies (302 IU/ml, normal <30 IU/ml). Screening for multiple myeloma and sarcoidosis (including angiotensin-converting enzyme level, complement levels, and immunoglobulin profile) were also negative. Cardiac MRI with gadolinium also revealed no areas of delayed enhancement and no evidence of myocarditis or infiltrative disease. During her stay in the hospital, the patient experienced repeated episodes of symptomatic bradycardia associated with transient episodes of complete heart block on ECG. A transvenous pacemaker was subsequently inserted and her symptoms resolved (Fig. 2). The monitor showed pacemaker dependency most of the time. The patient started on a strict gluten-free diet and was discharged home. On a follow-up visit after eight weeks, the patient was free of symptoms and her resting ECG showed a normal sinus rhythm. The interrogation of the pacemaker revealed a significant reduction of ventricular pacing periods compared to the discharge picture.
Figure 1. Complete heart block, third-degree atrioventricular block with a ventricular rate of 37 beats/minute
The recurrent symptoms with ECG manifestation of complete heart block in a patient with celiac disease and high specific antibodies titer with the absence of other possible aetiologies that can explain the conduction system abnormality, including the coronary artery disease, infiltrative disease, and a culprit medication raised the possibility of celiac disease-induced conduction system abnormality that manifested with complete hearty block. The improvement of symptoms and conduction abnormality after a gluten-free diet support the possible association.
DISCUSSION
Celiac disease is a malabsorptive multisystemic long-term autoimmune disorder characterized by inflammation and villous atrophy in the small bowel triggered by gluten ingestion in genetically predisposed individuals. The prevalence of celiac disease in the general population is around 1%, whereas in their first-degree relatives, it ranges between 5% and 20%[1] and often goes undetected due to varied and occult presentation that make diagnosis difficult. Celiac disease can present with intestinal and extraintestinal manifestations. The American College of Gastroenterology recommends screening for celiac disease in individuals with symptoms[2]. Intestinal symptoms related to malabsorption include loss motions, steatorrhea, anorexia, intermittent abdominal pain, abdominal bloating with distention, and weight loss. In adults, iron deficiency anaemia is the most common extraintestinal manifestation of celiac disease. A wide range of extraintestinal manifestations includes osteoporosis, malaise, aphthous stomatitis, headache, and reproductive disorders[2]. The diagnostic pathway of celiac disease includes the presence of high titer of immunoglobulin A (IgA) anti-tissue transglutaminase (tTG) antibody (IgAtTG) followed by certain histopathological findings on biopsies of the small intestine that can confirm the diagnosis of celiac disease[3]. A concomitant IgA deficiency may lead to false-negative serology results, and in these cases, the recommendation is to measure anti-tTG immunoglobulin G (IgG) level or anti-deamidated gliadin peptide IgG antibody levels[1]. A strict gluten-free diet is the treatment strategy that helps improve symptoms and decreases the risk of comorbidities without eliminating the condition[4].
A recent prospective analysis of a large-cohort study in the UK investigated whether people with coeliac disease are at increased risk of cardiovascular disease. It concluded that individuals with celiac disease had a higher risk of developing cardiovascular disease than those without it. However, they had a lower prevalence of traditional cardiovascular risk factors[5]. The association between celiac disease and certain cardiovascular pathology was found in many recently published studies[6]. The relationship between celiac disease and cardiomyopathy was discussed in the largest number of studies (around 33). The association of celiac disease with thrombosis, premature atherosclerosis, stroke, arterial function, and ischemic heart disease was also found in fewer reports. The changes in arterial wall stiffness and distensibility index were more prominent in patients with untreated celiac disease than controls[7], which may increase the risk for coronary artery disease[8]. Bayar et al. reported a case of acute myocardial infarction as a result of spontaneous multivessel coronary artery dissection in young patients with celiac disease[9]. The correlation between celiac disease and coronary artery disease or cerebrovascular disease was also supported by a meta-analysis performed by Heikkilä et al., but the evidence base was heterogeneous and had limitations[10]. The potentially elevated risk of early atherosclerosis in adults with celiac disease was also seen in a study where a vascular impairment and unfavourable biochemical risk pattern led to chronic vascular inflammation, and these abnormal findings were normalised with gluten abstinence[11]. In untreated celiac disease, the elevated level of homocysteine (hyperhomocysteinemia) and deficiencies in protein S, folate, and vitamin B2 may lead to thrombophilia with a subsequent elevated risk of thrombosis[12]. The sites of associated venous thrombosis are generally unusual in celiac disease. Two cases of cerebral venous thrombosis were reported in patients with celiac disease[13]. The risk of stroke has also been found to increase in untreated asymptomatic patients with celiac disease, which should be considered a possible aetiology in cases of stroke in youth with unknown causes[14]. The deficiency of vitamin B12 and folic acid, in addition to arterial vasculopathy and possible associated autoimmune disease like antiphospholipid syndrome, may explain the possible pathogenesis of stroke in celiac disease.
One serious and potentially fatal association with celiac disease is cardiomyopathy. The high prevalence of celiac disease has been noted with different types of cardiomyopathy, including dilated cardiomyopathy, idiopathic cardiomyopathy, and ischemic or valvular cardiomyopathy[15,16]. Not et al. looked at 238 patients with idiopathic cardiomyopathy and 418 of their relatives and concluded that celiac disease appears to be associated with but not co-segregated within familial cases[15]. The authors of the study suggested that iron deficiency anaemia as a co-morbidity in patients with dilated cardiomyopathy should raise the possibility of celiac disease association[15]. Based on the result of the Brazilian study that aimed to evaluate the prevalence of celiac disease among south Brazilian precardiac transplant patients with advanced dilated cardiomyopathy (total of 74 patients), celiac disease was reported in 12.8% of these patients. De Bem et al. recommended screening all patients with dilated cardiomyopathy for celiac disease[17]. Frustaci et al. showed a high prevalence of celiac disease among patients with idiopathic congestive heart failure and biopsy-proven myocarditis[18]. An autoimmune reaction directed against antigenic components may explain the cardiac tissue injury with a subsequent picture of myocarditis in patients with untreated celiac disease[18]. Immunosuppression and gluten free diet are often effective therapeutic options in celiac disease patients with heart failure manifestation as a result of myocarditis[18]. Elnour et al. reported a 33-year-old female with severe iron deficiency anaemia and dilated cardiomyopathy secondary to celiac disease, and they concluded that high clinical suspicion is needed to identify celiac disease in patients diagnosed with dilated cardiomyopathy and iron deficiency anaemia, as an improvement in cardiac function has been demonstrated with a gluten-free diet[19].
In a large cohort study, patients with proven celiac disease were at increased risk of atrial fibrillation, probably related to the elevation of inflammatory markers that predict atrial fibrillation[20].
Only a few cases documented the association of celiac disease with atrioventricular blocks in adults. In two different case reports, a combination of atrioventricular block in the presence of both celiac disease and idiopathic pulmonary hemosiderosis was documented[21,22]. In contrast, in two case reports, both patients had concomitant dilated cardiomyopathy with celiac disease and heart block[23,24]. One case report in the literature demonstrated an isolated atrioventricular block in a 42-year-old female who presented with syncope and high-grade atrioventricular block, and her workup revealed undiagnosed celiac disease and iron deficiency anaemia with no other concomitant pathology and her conduction abnormality improved five months after a gluten-free diet introduction[25]. A paediatric case of 4-year-old male with acquired progressive atrioventricular block who was diagnosed with celiac disease demonstrated a significant improvement of his conduction abnormality after a strict gluten-free diet. The authors of this case suggested screening for celiac disease in all children with acquired atrioventricular block[26].
A few case reports in the literature demonstrated a spontaneous recovery of advanced heart block without treatment. One case report involved an infant who had a complete congenital heart block in the third trimester spontaneously reverting to a normal sinus rhythm 5 days after birth without intervention or medication[27]. Kitazawa et al. reported a case of advanced atrioventricular block in the patient without any comorbidities after gynaecological surgery that may have been triggered by postoperative nausea, with spontaneous recovery[28].
CONCLUSION
The association of celiac disease with isolated advanced atrioventricular conduction abnormality is rare and a gluten-free diet may help improve the conduction abnormality.