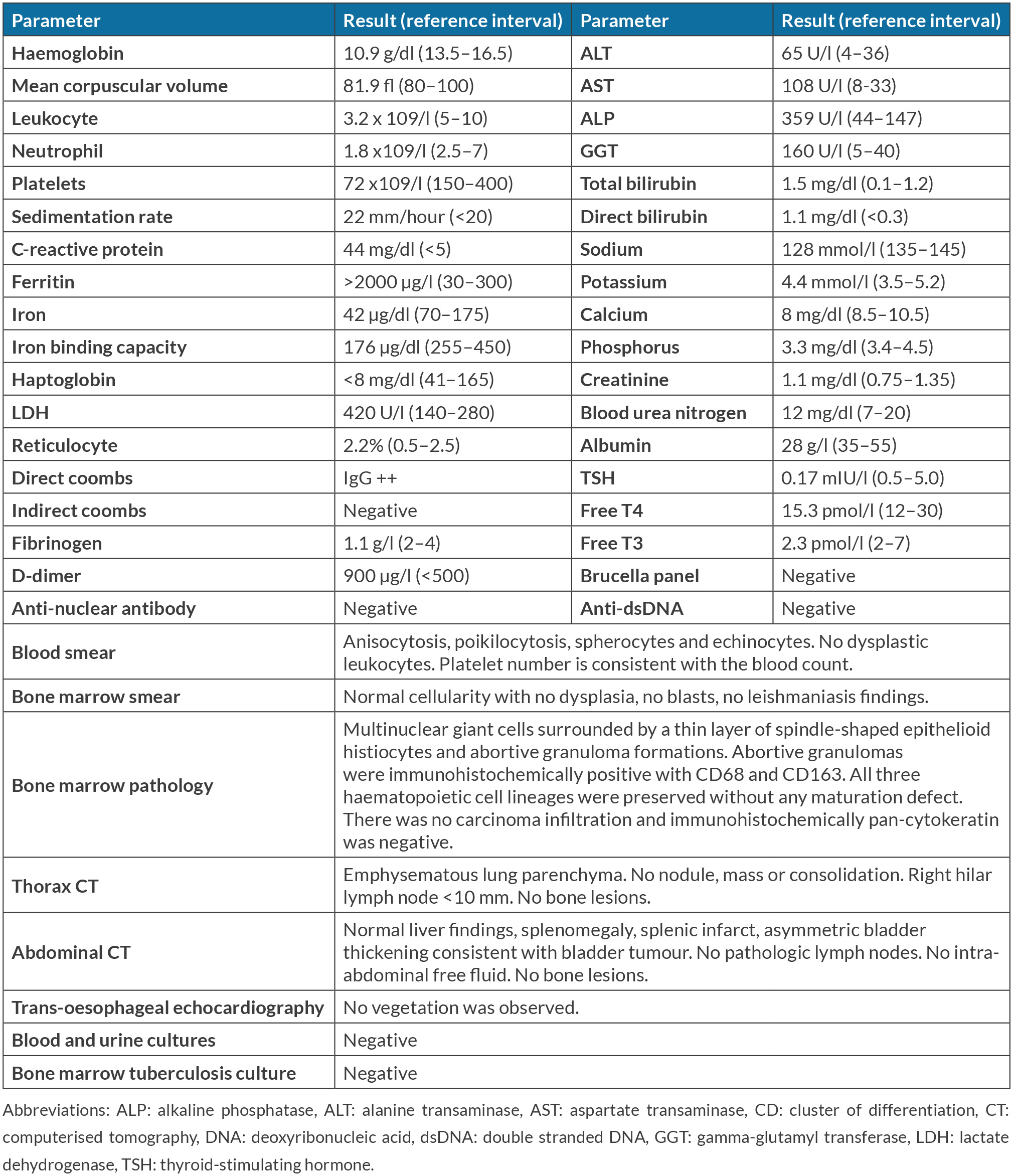
ABSTRACT
Intravesical bacillus Calmette-Guérin (BCG) is used for urothelial carcinoma. Systemic side effects are rare and commonly include organ involvement but rarely include bone marrow. We describe a patient who had received intravesical BCG and presented shortly afterwards with constitutional symptoms. Initial work-up revealed pancytopenia and immune haemolysis. He was presumptively diagnosed with systemic BCG infection and secondary warm autoimmune haemolytic anaemia. Isoniazid, rifampin and ethambutol was started. The bone marrow biopsy revealed granulomas. Within 6 weeks of treatment, the patient's clinic and laboratory results were dramatically improved. A high level of suspicion is crucial for diagnosis and treatment.
LEARNING POINTS
- Systemic bacillus Calmette-Guérin (BCG) infection following intravesical BCG instillation is a rare but serious consequence. A high level of suspicion and scrutiny of history is of paramount importance for diagnosis.
- Autoimmune haemolytic anaemia secondary to systemic BCG infection is even rarer.
- Autoimmune haemolytic anaemia resolution was in parallel with improvement in systemic BCG infection.
KEYWORDS
Mycobacterium bovis, urinary bladder neoplasms, anaemia, immunotherapy
INTRODUCTION
Bacillus Calmette-Guérin (BCG) is a live attenuated strain of Mycobacterium bovis. Intravesical BCG instillation is used for the treatment of superficial urothelial carcinoma. BCG acts as an immune stimulator, creating a local inflammatory response that targets malignant cells[1]. Although self-limiting minor side effects such as fever, dysuria and seldom occur, systemic and life-threatening side effects are extremely rare. Systemic side effects include disseminated BCG infection, which can be accompanied by pulmonary, renal, miliary and meningeal involvement as well as haemophagocytic syndrome[2,3]. Besides their rarity they are often hard to diagnose, and high clinical suspicion is of paramount importance due to the non-specific nature of symptoms. Furthermore, a pending culture result can lead to a profound treatment delay that may cause increased morbidity and mortality. We report a patient who received intravesical BCG immunotherapy for superficial bladder cancer and developed disseminated BCG infection and superimposed warm autoimmune haemolytic anaemia shortly after instillation.
CASE DESCRIPTION
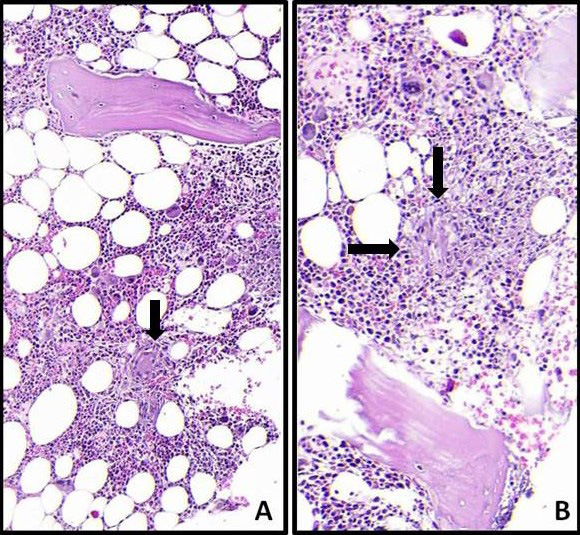
A 65-year-old male with a history of larynx carcinoma and low-grade papillary urothelial carcinoma being treated with intravesical BCG instillation was admitted to our institution's general internal medicine outpatient clinic. He reported recent involuntary weight loss of 15 kilograms, appetite loss, and sweating and severe fatigue that impeded his ambulation. He denied fever, pain, cough, sputum, dyspnoea, vocal changes, palpitation or a skin rash. On examination, his skin looked pale, and his muscles looked cachectic, but physical examination did not reveal any further findings. Vital signs were normal as well. His larynx carcinoma was cured five years ago. He had received three trimonthly BCG instillations in total and his last intravesical BCG therapy was two months previously, which was two weeks prior to the symptom onset. He did not report any daily medication, alcohol, or illicit drug use. Initial laboratory results revealed pancytopenia, severe hyperferritinemia, hyponatraemia and abnormal liver function tests. He was admitted to the general internal medicine ward for further diagnostic work-up. Table 1 shows the work-up and findings in detail. His clinical and initial laboratory findings suggested disseminated BCG infection; thus, empirical isoniazid, rifampicin and ethambutol were started. Bone marrow pathology and tuberculosis culture results were pending at the time of diagnosis and the start of treatment. Bone marrow pathology later showed multinuclear giant cells and abortive granuloma formations (Fig. 1). Warm autoimmune haemolytic anaemia was thought to arise secondary to the infection. Since his haemoglobin levels were above 10 g/dl and his platelets were over 50x109/l and regarding systemic BCG infection, he did not have organ failure, glucocorticoid therapy was not initiated. He was discharged with close outpatient follow-up. Two weeks after the therapy's initiation, his appetite and fatigue had improved. His haemoglobin levels were 12.1 g/dl and his platelets were 203x109/l. Liver function test results were markedly improved as well. His anti-mycobacterial therapy was continued without any side effects. At the end of the six weeks he had started to gain weight, his appetite and fatigue were markedly improved and he could ambulate independently. His laboratory results were as follows: haemoglobin: 12.9 g/dl, platelets: 168x109/l, ALT: 17 U/l, AST: 28 U/l, CRP: 23 mg/dl Since his condition improved without any side effects, the medications were continued and a monthly visit was planned.
Figure 1. Microphotograph reveals a multinucleated giant cell surrounded by a thin rim of epithelioid histiocytes (A, single arrow, H&E x200) and an abortive granuloma (B, double arrows, H&E x200)
DISCUSSION
Intravesical BCG immunotherapy is indicated for patients with intermediate- to high-risk non-muscle-invasive urothelial bladder cancer following transurethral resection of the bladder tumour (TURBT). While not fully elucidated, our current knowledge regarding the mechanism of intravesical BCG includes but is not limited to increased interferon gamma expression, increased cytokine levels and the induction of CD4 T cells and macrophage infiltration within the bladder[4]. Intravesical BCG consists of high molecular weight compounds; thus, high local concentrations of immunotherapy are achieved without marked transmucosal absorption, and systemic toxicity is largely eliminated. However, several risk factors have been described for systemic BCG infection following instillation and include traumatic catheterisation, active cystitis, persistent and gross haematuria following TURBT, immunosuppression, and age over 70 years[5-7]. BCG infection can manifest as either an early-onset infection or a delayed-onset infection. Early-onset BCG infection usually presents with constitutional and systemic symptoms, whereas delayed-onset BCG infection usually presents with localised disease. Disseminated BCG infection is the most commonly reported form of systemic BCG infection, and it presents as miliary disease, sepsis or organ involvement. Commonly involved systems include the pulmonary, hepatic, musculoskeletal and vascular systems, and rarely the bone marrow[8,9]. A review that included 282 patients with BCG infection revealed no bone marrow involvement[8]. Bone marrow involvement is exceedingly rare, but case reports are similar to our patient's findings. Of the 10 patients with systemic BCG infection and bone marrow involvement, all had constitutional symptoms and pancytopenia similar to our patient[9]. Differing from our patient, none reported warm autoimmune haemolytic anaemia. Although reticulocytosis is expected during the course of haemolytic anaemia, our patient's reticulocyte was in the normal range. This is due in part to the accompanying anaemia of inflammation.
Since Mycobacterium bovis is resistant to pyrazinamide, treatment consists of isoniazid, rifampin and ethambutol for 2 months and then isoniazid and rifampin for 7 months. Although glucocorticoid administration concomitant with antimycobacterial therapy in the setting of extensive miliary involvement and/or respiratory failure can be considered, we did not administer glucocorticoids due to a lack of respiratory failure and non-extensive involvement. Haemoglobin and thrombocyte levels were also not low enough to justify glucocorticoids. Considering the patient's muscle wasting and lack of ambulation, the potential risks of glucocorticoids seemed to outweigh the potential benefits as well. Therefore, we proceeded with triple antimycobacterial therapy without glucocorticoid therapy and close monitoring for a possible glucocorticoid need, but the patient's clinical status and laboratory results improved without the need for glucocorticoid.
Once a patient is correctly diagnosed and therapy is initiated, attributable mortality rates are 5.4% and long-term morbidity is 7.4%[8]. Due to the rarity and non-specific nature of signs and symptoms, a high level of suspicion is needed to diagnose a systemic BCG infection.