ABSTRACT
Central nervous system (CNS) lymphoma is a rare and aggressive primary neoplasm that comprises a small proportion of brain tumours and non-Hodgkin lymphomas. We present a case report of a 64-year-old woman with CNS lymphoma, who exhibited cognitive changes, weight loss and neurological symptoms. Imaging scans revealed multiple lesions in the brain and thrombosis in the venous sinuses. A diagnosis of diffuse large B-cell lymphoma of the CNS was confirmed through histological examination. The patient underwent treatment with corticosteroids and chemotherapy, but experienced clinical deterioration with thrombocytopenia and disease progression. Despite efforts to manage complications and provide targeted therapy, the patient passed away. Primary CNS lymphoma typically responds well to chemotherapy, and prognostic factors such as age and functional status play a significant role in patient outcomes. However, complications such as thromboembolism pose challenges during treatment due to the hypercoagulable state induced by chemotherapy agents. The pathophysiology of thromboembolic events in the context of malignancy remains uncertain but may involve direct tumour compression, vascular invasion and alterations in coagulation factors. The diagnostic process for CNS lymphoma can be complex, and the information obtained from cerebrospinal fluid analysis, including flow cytometry, may be limited in cases with low cell counts. Ongoing research exploring genetic tests and biomarkers shows promise for improving diagnostic accuracy in such cases. This case underscores the need for comprehensive management strategies that address both the neoplasm and its associated complications, to optimise patient outcomes.
LEARNING POINTS
- Primary CNS lymphoma is a rare primary neoplasm, being even rarer in immunocompetent patients.
- In 25% of all cases of CNS lymphoma, it is complicated by cerebral venous thromboembolism.
- Leptomeningeal spread can occur with or without MRI evidence and is diagnosed with cerebrospinal fluid (CSF) flow cytometry. Age and functional status are important prognostic factors.
KEYWORDS
Central nervous system lymphoma, cerebral venous sinus thrombosis, thromboembolism, hypercoagulability, refractory neoplasm
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive primary neoplasm, accounting for only 2% of all brain tumours, and 4–6% of non-Hodgkin lymphomas (NHL). The incidence rate is approximately 0.45 per 100,000 people[1]. PCNSL in immunocompetent patients is rare and represents 4% of all intracranial neoplasms and 4% to 6% of all extranodal lymphomas. The mean age of diagnosis is around 67 years; it is more prevalent in men and increases with age. In 25% of all cases, it is complicated by cerebral venous thromboembolism, with its occurrence being more common during the diagnosis phase and early treatment, and its prevalence being higher in large B-cell NHL[2]. The treatment is similar to that of other causes of cerebral venous thrombosis (CVT). Direct oral anticoagulants (DOACs) play an uncertain, but apparently non-inferior role when compared to other anticoagulant therapies; however, there are still no studies validating DOACS in cancer with thrombosis in atypical locations[3].
CASE PRESENTATION
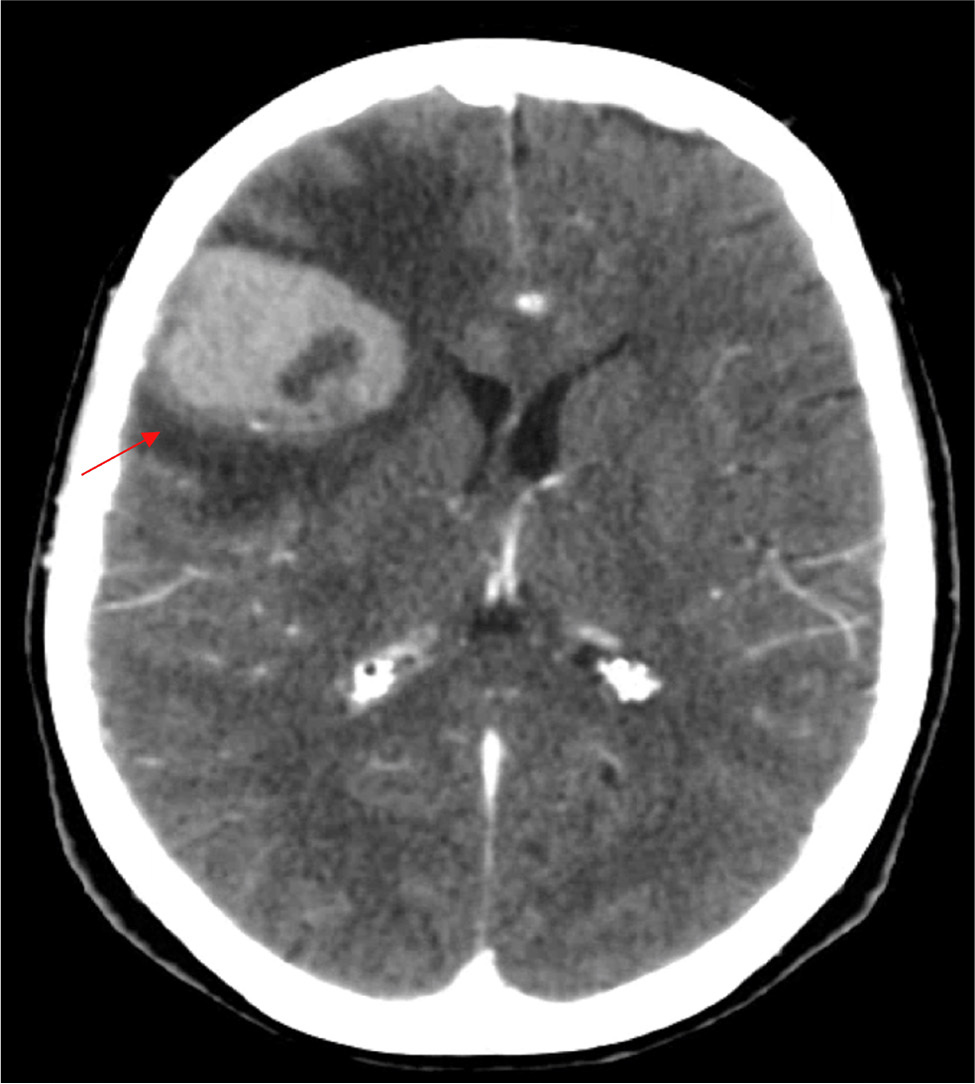
The patient is a 64-year-old woman with an Eastern Cooperative Oncology Group (ECOG) score of 2, a medical history of dyslipidemia and myofascial syndrome with hypersensitivity, with pain controlled pharmacologically, autoimmune thyroiditis managed with supplementation, and a hysterectomy for uterine leiomyomatosis. She was admitted in the emergency room due to cognitive changes that had been evolving for three months, along with constitutional symptoms including weight loss of 10 kg (16% of her body weight) over a period of 9 months. On neurological examination, she presented with apathy, temporal and spatial disorientation, difficulty with naming and paraphasia, hesitation in carrying out complex commands, ideomotor apraxia and mild right-sided dysmetria. A cranioencephalic computed tomography (CT) scan showed multiple supra- and infratentorial lesions with contrast enhancement, some with vasogenic oedema causing mass effect, without hydrocephalus (right frontal lobe, left inferior temporal pole, left internal intralenticular region and right cerebellar hemisphere) (Fig. 1).
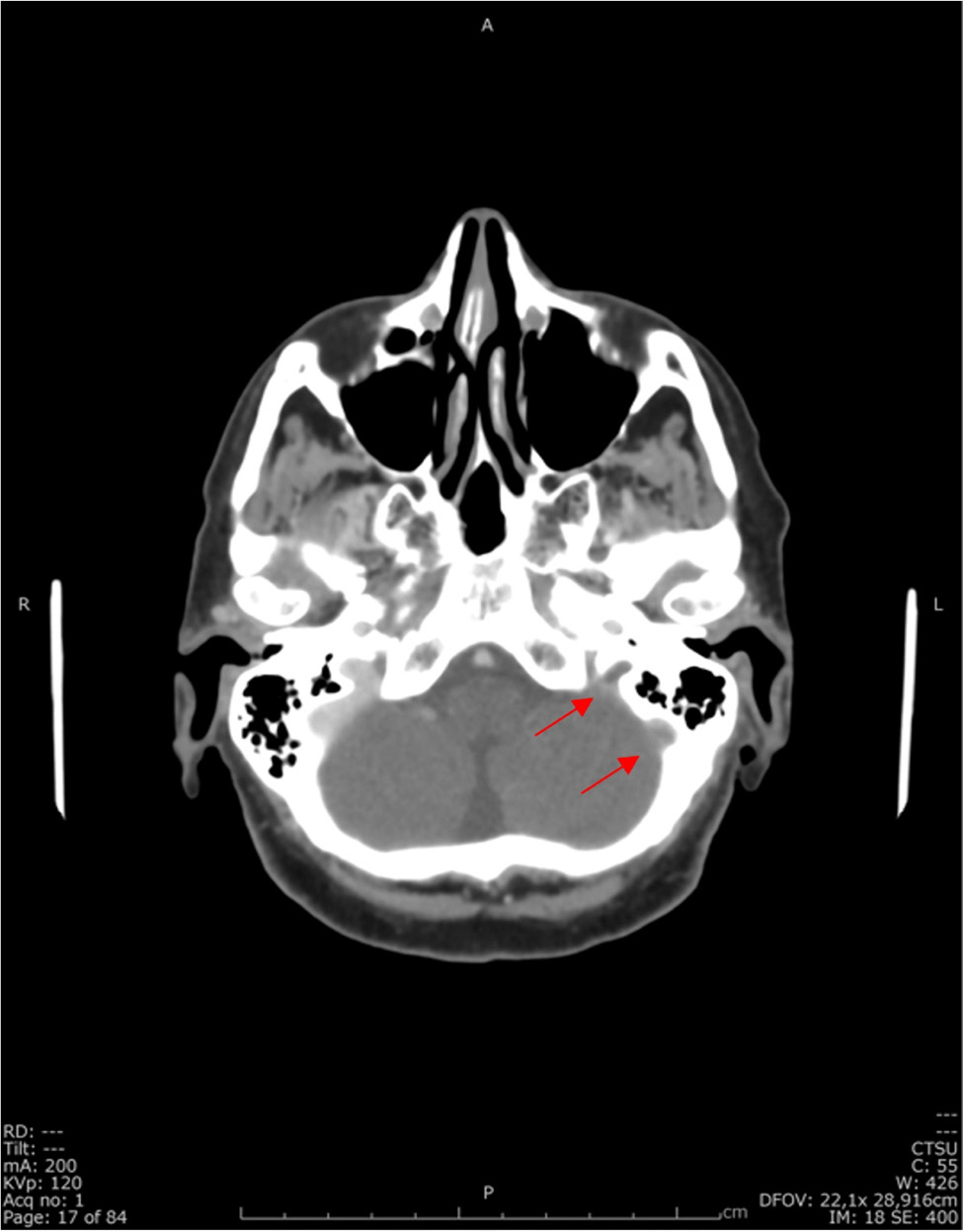
Additionally, the patient had thrombosis of the internal jugular vein, sigmoid sinus and the proximal portion of the left transverse sinus (Fig. 2).
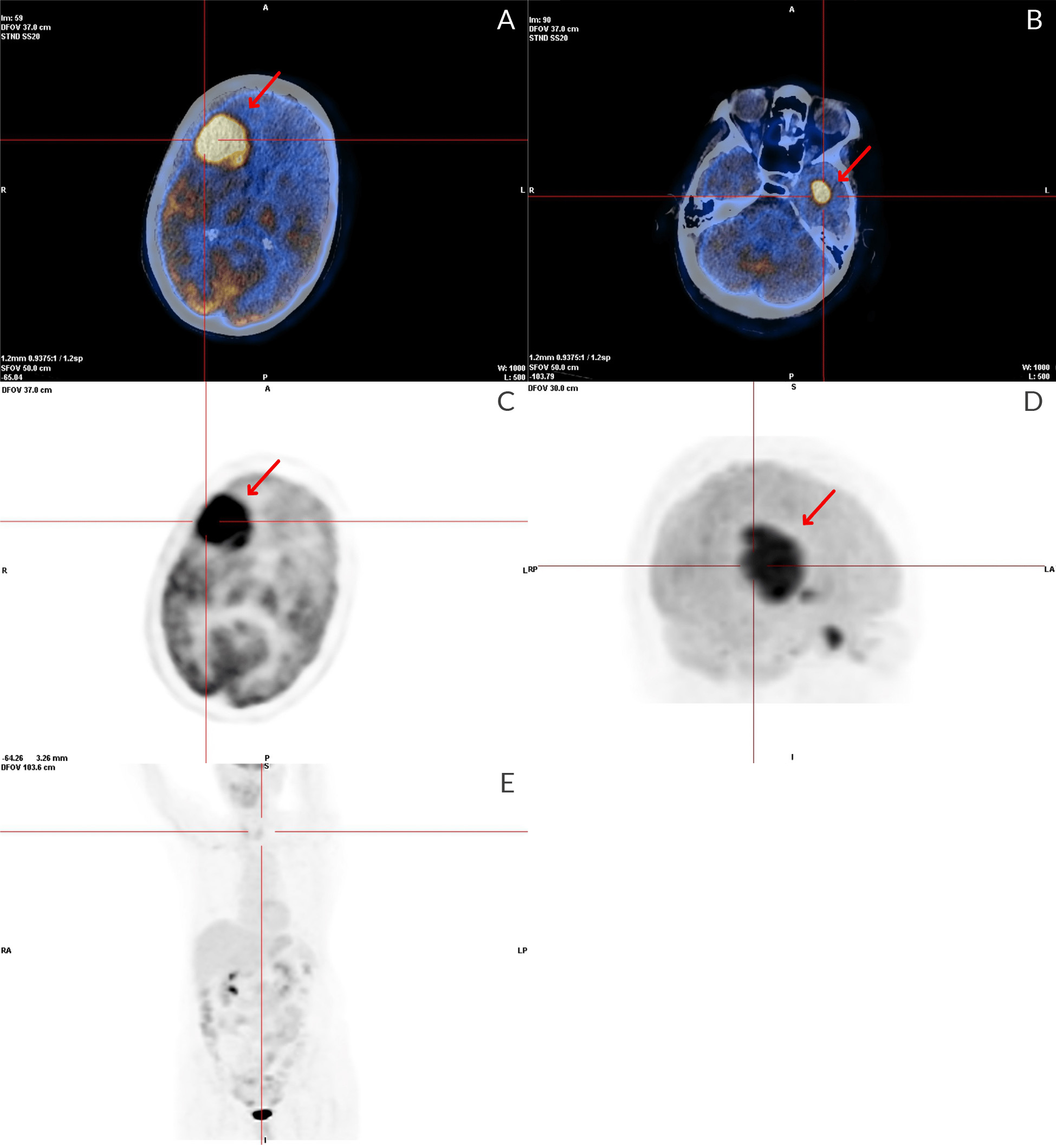
An investigation for occult malignancy, including body FDG-PET (Fig. 3), mammography, endoscopic studies and gynaecologic ultrasound, were negative.
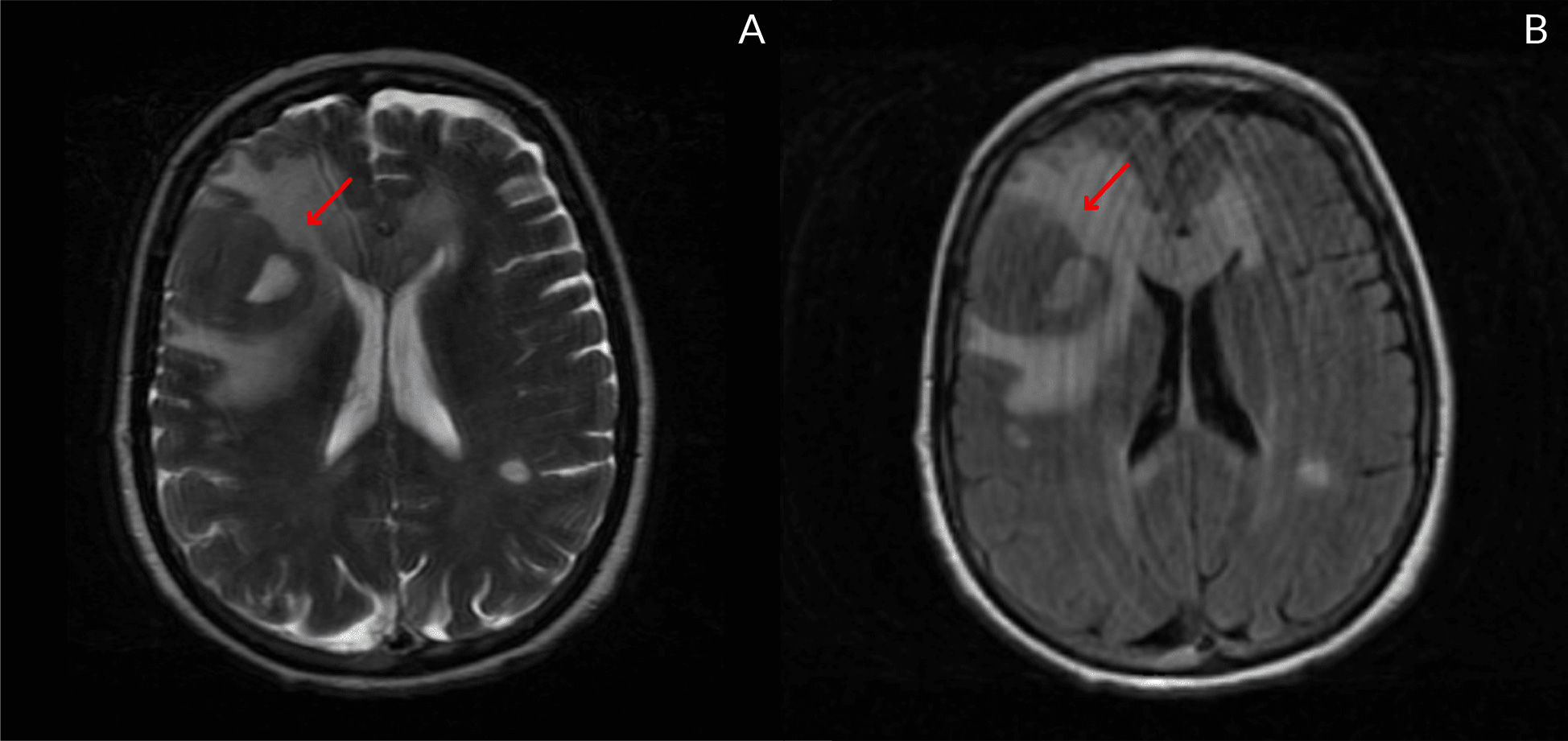
Further evaluation with magnetic resonance imaging (MRI) revealed similar additional lesions, raising the suspicion of metastatic lesions or a lymphoproliferative nature (Fig. 4).
Figure 1. Cranioencephalic CT: right frontolateral neoplastic lesion with extensive oedema halo (red arrow)
Figure 2. Cranioencephalic CT venogram: absence of contrast filling the sigmoid sinus and jugular bulb (red arrows)
Figure 3. FDG-PET SCAN: In the cerebral study, three foci of intense hypermetabolism were observed; respectively, in a large mass in the right frontal lobe, near the left caudate nucleus, and in the left temporal lobe. It also refers to an area of hypometabolism involving the frontal lobes, probably related to oedema. In the whole-body study, foci with abnormal and significantly increased uptake of F18-FDG suggestive of malignant infiltration of high glycolytic metabolic grade are not visible
Figure 4. cortico-subcortical right frontal lateral lesion. On T2 sequences, they appear with irregular morphology and well-defined boundaries that tend to be hypodense, enhancing homogeneously and intensely after contrast. There is surrounding vasogenic oedema around the right frontal lesion (measuring 37x34x33 mm)
The patient was started on systemic corticosteroid therapy (dexamethasone 24 mg/day) and anticoagulation with weight-adjusted enoxaparin, later replaced by edoxaban 60 mg/day. Cerebrospinal fluid (CSF) analysis showed 10 cells, predominantly lymphocytes, with normal protein levels (35.5 mg/dl); immunophenotyping did not show any abnormalities. Brain biopsy was performed by neurosurgery in another hospital, and histological examination revealed morphological and immunophenotypic characteristics consistent with diffuse large B-cell lymphoma of the central nervous system (CNS) (images not available). The patient was referred for a haemato-oncology medical appointment and was proposed treatment with dexamethasone, rituximab, high-dose methotrexate and cytarabine. Despite initial clinical improvement, on the twentieth day of chemotherapy there was subsequent fluctuation in consciousness, marked thrombocytopenia (<20,000/μl) requiring transfusion, new venous thrombotic events and progression of neoplastic lesions. Palliative symptomatic treatment was decided upon, and the patient passed away less than three months after diagnosis.
DISCUSSION
The annual incidence of PCNSL in the United States is 7 in 1,000,000, representing approximately 4% of CNS tumours. Secondary involvement occurs primarily in aggressive B-cell lymphomas and is present in approximately 5% of cases[4]. Acquired or congenital immunodeficiency is the best-established risk factor, and the disease usually presents as a single tumour located mainly in the frontal lobes (20%–43%) and the basal nuclei (9%–13%)[5].
PCNSL usually responds positively to chemotherapy with a mean survival of over 10 years. Age and functional status are important prognostic factors. Diagnosis and treatment of complications such as thromboembolism are mandatory, as the chemotherapy used increases the risk of new events: methotrexate, due to increased serum homocysteine levels, and corticosteroids, due to impaired fibrinolysis. Despite this state of hypercoagulability, the risk of intracranial haemorrhage is also high, especially when associated with thrombocytopenia, as in the case of our patient[6].
The pathophysiology of CVT in the context of malignancy is uncertain. Potential mechanisms include direct compression by the tumour, vascular invasion and a state of hypercoagulability related to increased expression of tissue factor (procoagulant) and inflammatory cytokines, as well as dysregulation of protein C and thrombomodulin.
The diagnostic process in this patient was difficult. Initially, the search for a primary occult neoplasm was performed, due to the suspicion of brain metastases, without success. The study of the cerebrospinal fluid did not guide in any other way. Lesions caused by PCNSL have higher metabolic activity than metastases and high-grade gliomas with high FDG rates in PET, as seen in this case. Leptomeningeal spread can occur with or without MRI evidence and is diagnosed with CSF flow cytometry[7]. The result of the CSF cytometry study in this case was not informative, perhaps because of the low cell count. Some studies with genetic tests and measurement of other biomarkers such as IL-10 in CSF are under development and showing favourable results[8]. The fact that the occult neoplasm study was negative combined with the MRI results were crucial to initiate the diagnostic suspicion that was later confirmed with biopsy.
In addition to imaging tests, when there is a high diagnostic suspicion of PCNSL, a biopsy of the lesion with a histopathological study is the gold standard for the diagnosis of the disease and the establishment of an appropriate therapeutic plan[5].
As it is an aggressive disease, it is important to develop prognostic models and three are described:
- The International Extranodal Lymphoma Study Group (IELSG) scoring system is composed of five prognostic variables (age more than 60 years, ECOG performance status higher than one, elevated serum LDH level, high CSF protein concentration, and involvement of the deep regions of the brain e.g., brain stem, cerebellum).
- The Nottingham/Barcelona (NB) model is composed of three risk factors (age equal to or greater than 60 years, ECOG greater than one and the presence of multifocal lesions).
- The Memorial Sloan-Kettering Cancer Center (MSKCC) model uses only two variables: age and Karnofsky performance status)[9,10].
There is still no consensus on the ideal model to adopt. Our patient had an IELSG score of 4, which defined a survival prognosis of 15% at 2 years.
Despite all efforts, the delay in diagnosis may have compromised the success of the first-line therapy and the prolonged stay at the hospital worsened the initial status, which did not allow more invasive therapeutic approaches.
The unfavourable outcome in the short term could also be explained by the refractory nature of the neoplasm to targeted therapy. In these cases of resistance to first-line chemotherapy, the evidence of improvement to alternative therapies is poorer.
CONCLUSION
In conclusion, this case report highlights the challenges associated with the diagnosis, treatment and management of diffuse large B-cell CNS lymphoma complicated by cerebral venous sinus thrombosis. Venous thromboembolism is a common complication in CNS lymphomas, particularly during the diagnosis phase and early treatment, with a higher prevalence in large B-cell NHL. The pathophysiology of cerebral venous sinus thrombosis in the context of malignancy remains uncertain but is probably multifactorial, related to the disease and treatment agents.
This case underscores the importance of early recognition and appropriate management of thrombotic complications in CNS lymphoma. Further studies are warranted to enhance our understanding of the pathogenesis, refine diagnostic approaches and develop more effective targeted therapies to improve outcomes for patients with this rare and aggressive neoplasm.