ABSTRACT
Marijuana has long been used both for recreational and medicinal purposes. Most of the available forms of marijuana contain additives such as liquorice to enhance its flavour. Liquorice increases the amounts of cortisol in the body and produces metabolic abnormalities seen in primary hyperaldosteronism[1]. Liquorice extracts are mixed with marijuana in the same way as for tobacco[2,3]. We describe a case of apparent mineralocorticoid excess due to excessive smoking of liquorice-laced marijuana. To our knowledge, this is the first reported case of apparent mineralocorticoid excess caused by marijuana use.
LEARNING POINTS
- First report of liquorice-laced marijuana causing syndrome of apparent mineralocorticoid excess (SAME).
- Since its legalisation, marijuana is reported to be the most used substance second only to alcohol. With the increased availability of marijuana, the health care providers and consumers must be cognisant of its side effects.
- It is a dose-dependent phenomenon. The effects vary from minor clinical symptoms to fatal arrhythmias based on the amount consumed.
KEYWORDS
Licorice, liquorice, marijuana, mineralocorticoid
CASE REPORT
A 48-year-old female with no reported medical history was brought to the emergency department (ED) for the evaluation of hypertension. The patient complained of generalised body aches and weakness of 2 weeks duration. She reported daily consumption of alcohol and marijuana (in the form of smoking) for the previous 3 weeks in addition to 15-pack years of cigarette smoking. She denied over-the-counter drug use, herbal supplement and diuretic/laxative use.
Initial vitals in the ED showed BP of 205/111 mmHg and pulse of 100/min. She was a lean woman who had a depressed affect. The remainder of the physical exam was unremarkable.
Initial diagnostic investigations revealed a potassium (K) level of 1.9 mmol/l, chloride 92 mmol/l and bicarbonate of 32 mmol/l. Creatine phosphokinase (CPK) was 1164 U/l and the urine drug screen was positive for cannabis. Urine K was 20.4 mmol/l and urine creatinine was 1.06 g/l. The urine K/creatinine ratio of 19 was suggestive of renal potassium wasting. Plasma renin and aldosterone levels were then ordered in the ED to confirm the diagnosis of hyperaldosteronism.
She was treated for hypertensive urgency with hydralazine 5 mg IV, amlodipine 10 mg and spironolactone 100 mg bid. Spironolactone was initiated as empiric treatment of hyperaldosteronism due to persistent hypokalaemia. For rhabdomyolysis, normal saline with potassium supplementation was given.
Repeat urine chemistries after the initiation of spironolactone confirmed a decrease in urine K from 20 mmol/l to 7 mmol/l, confirming initial renal K loss. In 2 days, the CPK improved to 400 U/l. Potassium was aggressively supplemented via intravenous and oral routes based on serial metabolic panels but remained persistently low, despite spironolactone. Contrast CT of the abdomen and pelvis did not demonstrate a renal or adrenal mass, or a renal artery stenosis. On day 3, losartan 50 mg was initiated. On day 4, potassium levels normalised and intravenous supplementation was discontinued.
On day 6, the serum potassium level was 5.3 mmol/l. The dose of spironolactone was decreased and later discontinued, on day 7 of hospitalisation.
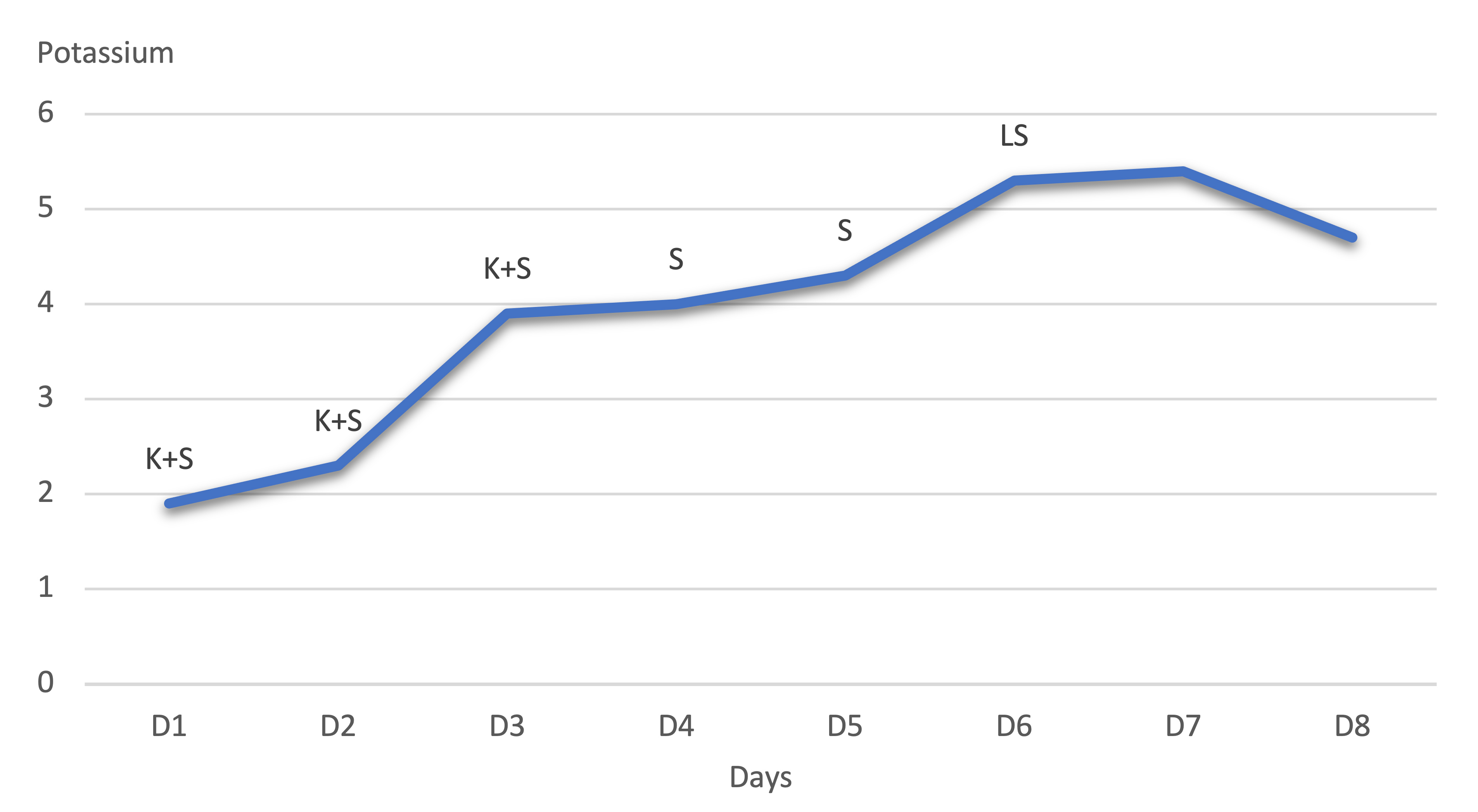
The following graph shows the potassium level trend during the hospital course (Fig. 1).
Figure 1. Potassium level trend during the hospital course. Abbreviations: potassium + spironolactone, K+S; spironolactone alone, S; low dose spironolactone, LS
The K levels remained normal from day 8 onwards. In the meantime, the patient’s blood pressure improved on amlodipine 10 mg daily, losartan 50 mg daily.
Interestingly, result of the serum aldosterone level ordered on the day of admission was <1 ng/dl. This ruled out the diagnosis of hyperaldosteronism and instead pointed towards an alternative diagnosis, which resolved within a week. The syndrome of apparent mineralocorticoid excess (SAME) fits the scenario. We speculate that in this case the SAME was caused by marijuana that contained liquorice as an additive. The patient was discharged on amlodipine 10 mg and hydralazine 50 mg three times daily, which was subsequently changed to hydrochlorothiazide 12.5 mg daily in clinic follow-up.
DISCUSSION
In this report, we describe a patient who was found to have profound hypokalaemia due to renal potassium loss, chloride-resistant metabolic alkalosis and uncontrolled hypertension, i.e. the symptoms of hyperaldosteronism. But her serum aldosterone level of <1 ng/dl excluded the diagnosis of primary aldosteronism. The temporary nature of her symptoms, evident by the complete resolution of her metabolic abnormalities during the week of hospitalisation, ruled out Liddles syndrome and ectopic ACTH production. The only remaining differential was acquired SAME caused by liquorice intake.
However, our patient denied any liquorice use. Upon further questioning, it was found that she was continuously smoking large amounts of marijuana. The literature review reveals that certain types of marijuana contain liquorice as a flavouring agent. We speculate that our patient was using liquorice-laced marijuana.
In 2021, recreational use of marijuana was legalised in New Jersey. Marijuana has different strains such as El Muerte, AK-47, Trainwreck and Liquorice Kush. The strain Liquorice Kush contains a significant amount of liquorice[4].
Liquorice is derived from the roots of Glycyrrhiza Glabra. It has various types: typica is the European variety, and in China and Western Asia varieties such as violacea, uralensis and pallidiflora are grown[5]. Ammonium glycyrrhizate and monoammonium glycyrrhizinate are the two liquorice derivatives that are bioavailable. Glycyrrhizin is hydrolysed into glycyrrhizinic acid (GA) by intestinal bacteria. GA is then absorbed into the bloodstream[6]. It inhibits 11β-hydroxysteroid dehydrogenase. As a result, the metabolism of cortisol to cortisone is inhibited, and increased levels of cortisol are free to bind the mineralocorticoid receptor and produce metabolic abnormalities, the same as hyperaldosteronism[7]. Eventually, GA is secreted in bile. A prolonged intake of liquorice can maintain dangerously high blood levels producing undesirable metabolic effects in a dose-dependent manner[8].
Commonly known sources of liquorice are candy, the Egyptian drink ‘Erk Sous’, Belgian beers, chewing tobacco, other tobacco products and tea[9-11]. Our patient denied the consumption of these. However, her urine drug screen was positive for cannabis. Although the patient was not tested for liquorice, this is the most plausible explanation of her symptoms.
The extract of liquorice has been claimed to have antiviral and anti-inflammatory effects, particularly for hepatitis and COVID-19[12]; it is a major part of Indian and Chinese herbal medicine[13]. Liquorice-infused marijuana products available include Black Licorice Bites, Ruby Fruits Licorice and Emerald Sky Edibles. Since liquorice is a part of many products of daily use, it is consumed by many people, but not all present with the symptoms of SAME. There seems to be an individual variation in sensitivity to liquorice. A regular intake of 100 mg/day of glycyrrhizic acid is considered the lowest dose causing adverse effects. The Japanese government and Dutch Nutrition Bureau recommend against glycyrrhizin consumption of more than 200 mg/day. The EU Scientific Committee advises against consumption more than 100 mg/day[5]. Hence it is proposed that the liquorice-induced apparent mineralocorticoid excess syndrome occurs only after chronic and continuous liquorice intake[14].
Unfortunately, we were not able to identify the type of marijuana our patient consumed. As several marijuana products contain liquorice as an additive, we propose that excessive consumption of liquorice-laced marijuana in a dose-dependent manner led to metabolic abnormalities in this case.