ABSTRACT
Long-acting (LA) cabotegravir/rilpivirine (CAB/RPV) is a complete regimen for the management of human immunodeficiency virus type 1 (HIV-1) infection to replace their oral antiretroviral therapy (ART) when they have been virologically suppressed. We present a case of successful achievement of undetectable HIV RNA viral load levels in an acquired immunodeficiency syndrome (AIDS) patient with long-standing virologic failure within two months of CAB/RPV LA initiation. This was later complicated by immune reconstitution inflammatory syndrome (IRIS) due to Mycobacterium avium-intracellulare (MAI) infection and hepatitis B virus (HBV) reactivation.
LEARNING POINTS
- This case highlights the efficacy of monthly CAB/RPV LA in rapidly reducing the HIV viral load level in a poorly controlled patient who lacked significant resistance to the two drugs.
- This is the first case of IRIS reported in the literature while using CAB/RPV LA. IRIS in the setting of occult MAI is well recognised. It would have occurred with good adherence to any regimen which rapidly suppressed the viral load and is unlikely to be due to CAB/RPV. CAB/RPV has no activity against HBV, which may have contributed to its reactivation.
- The patient had serologic evidence of resolution of prior HBV. However, some patients have covalently closed circular DNA (cccDNA) that may remain long term in hepatocyte nuclei.
KEYWORDS
Cabotegravir/rilpivirine, immune reconstitution inflammatory syndrome, IRIS, AIDS, HIV
INTRODUCTION
Long-acting (LA) cabotegravir/rilpivirine (CAB/RPV) is a complete regimen for the management of human immunodeficiency virus type 1 (HIV-1) infection in patients aged 12 years and older, with a minimum weight of 35 kilograms. It replaces their oral antiretroviral therapy (ART) when they have been virologically suppressed defined as HIV-1 ribonucleic acid (RNA) less than 200 copies/ml with no known or suspected resistance or treatment failure to CAB or RPV. It is administered as two intramuscular injections every one or two months.
We present a case of successful achievement of undetectable HIV RNA viral load levels in an acquired immunodeficiency syndrome (AIDS) patient with long-standing virologic failure within two months of CAB/RPV LA initiation. This was later complicated by immune reconstitution inflammatory syndrome (IRIS) due to Mycobacterium avium-intracellulare (MAI) infection and hepatitis B virus (HBV) reactivation.
CASE DESCRIPTION
A 47-year-old male infected with human immunodeficiency virus (HIV) type 1 with long-standing AIDS, cured hepatitis C virus, resolved HBV infection, remote neurosyphilis, cocaine abuse, migraine, chronic back pain with chronic narcotic use and chronic skin excoriations presented to the Infectious Diseases clinic for a follow-up visit. He had no known prior history of opportunistic infections.
The patient had been prescribed etravirine 200 mg, dolutegravir 50 mg, darunavir 600 mg and ritonavir 100 mg, all dosed twice daily by mouth with numerous resistance tests performed over the last several years showing no resistance to the current regimen. He did harbour HIV drug resistance mutations on old resistance testing to tenofovir, lamivudine, emtricitabine, abacavir and efavirenz. The patient was also prescribed Pneumocystis jirovecii pneumonia (PCP) prophylaxis with daily trimethoprim-sulfamethoxazole 800–160 mg and MAI infection prophylaxis with azithromycin 1200 mg every week.
On examination, the patient was mildly tachycardic, with a heart rate of 102 beats per minute. Otherwise, he had normal vital signs. Other relevant findings included a cachectic build with skin excoriations on the arms and legs. Despite stated compliance with his medications, his helper T cell count (CD4) was less than 10 cells/mm3 (normal 443–1471 cells/mm3), and his HIV viral load was more than 300,000 copies/ml. His CD4 count had been below 50 cells/mm3 for almost three years. Serum darunavir levels were obtained on several occasions in the past, which were not detectable or barely detectable on one occasion, indicating little to no medication was present in the patient’s system raising compliance concerns. Despite this, the patient mentioned that he was taking all his medications.
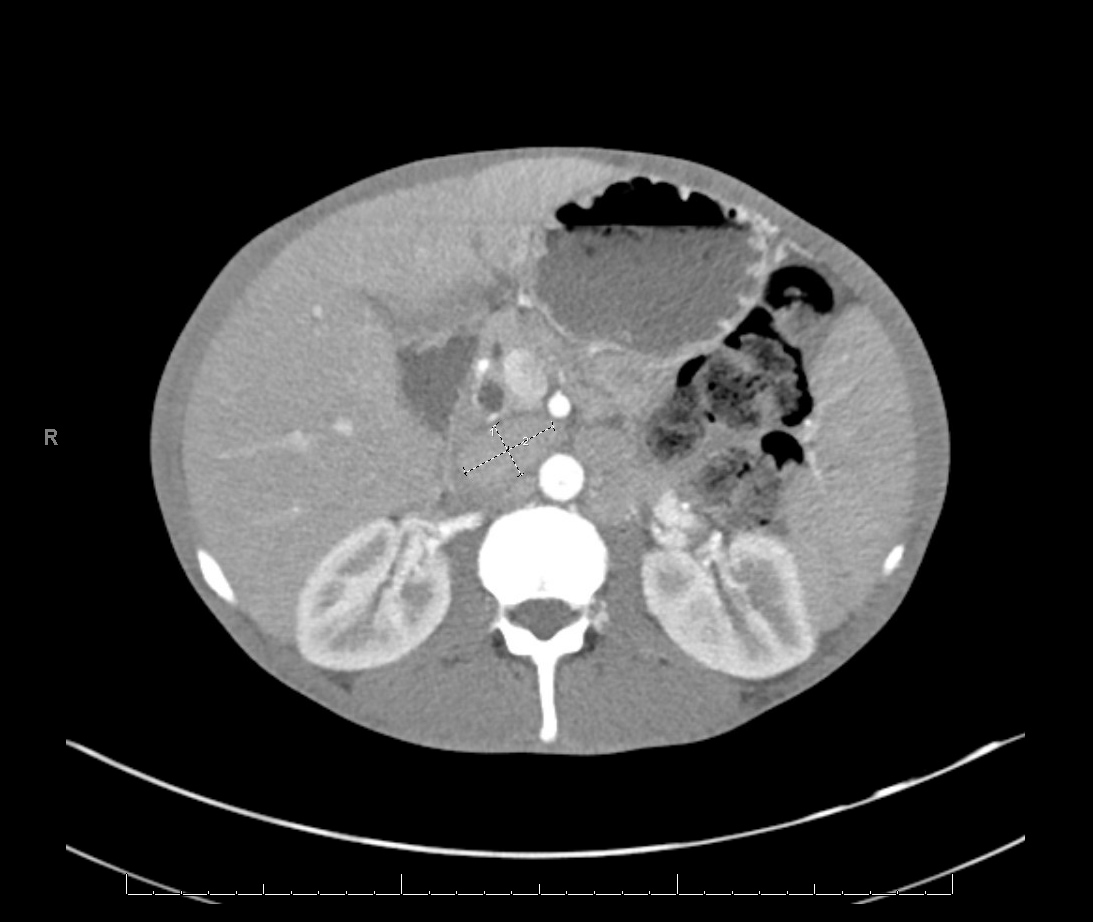
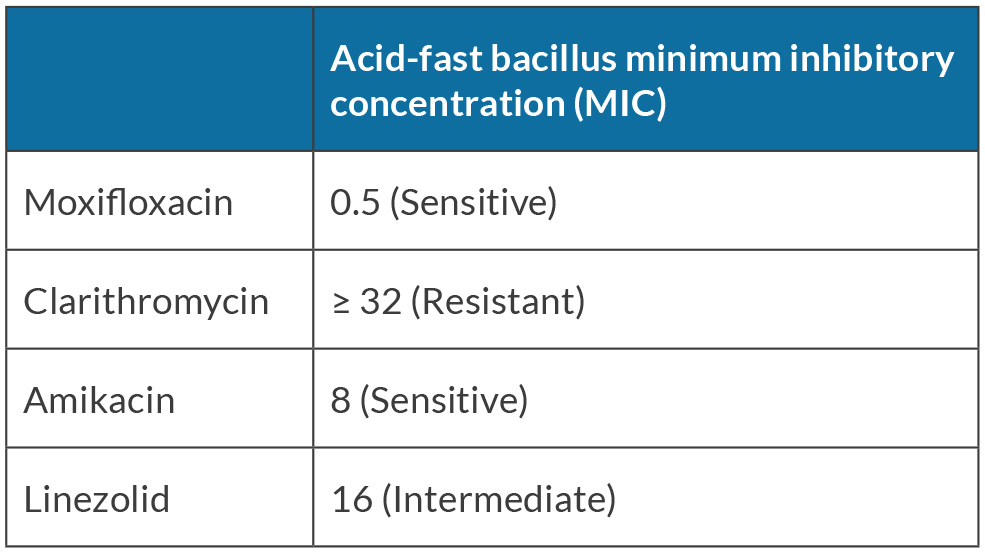
He became increasingly despondent and enrolled in a hospice due to his chronic pain and AIDS diagnosis. Given his decline and eventual progression to death without proper treatment, a decision was made to initiate a therapeutic trial of long-acting injectable cabotegravir/rilpivirine (CAB/RPV) in an attempt to induce virological suppression, despite a detectable viral load. He had no known resistance to either drug or had never taken either in the past. The patient received loading doses of CAB 600 mg and RPV 900 mg intramuscularly with no immediate adverse reactions. He was instructed to follow up monthly for maintenance dose administration of CAB 400 mg and RPV 600 mg, which he complied with. On follow-up, his HIV viral load became undetectable (less than 40 copies/ml), down from more than 300,000 copies/ml in less than two months. His CD4 count remained low at 21 cells/mm3. The patient had not had an undetectable viral load since his initial HIV diagnosis in 1996 and his liver transaminases were noted to be mildly elevated. The patient has a history of resolved hepatitis B (hepatitis B surface antigen [HBsAg] negative, hepatitis B surface antibody [anti-HBs or HBsAb] negative, hepatitis B core antibody [anti-HBc] positive, hepatitis B e-antigen [HBeAg] negative, hepatitis B e-antibody [HBeAb] positive, and HBV deoxyribonucleic acid [DNA] negative). Due to his history, repeat HBV DNA, HBsAg and HBeAg testing were performed and were all positive indicating HBV reactivation. The patient was started on emtricitabine 200 mg/tenofovir alafenamide 25 mg once daily by mouth. Computed tomography (CT) of the abdomen and pelvis was obtained due to fever, abdominal pain and distention, which revealed hepatosplenomegaly with multiple enlarged retroperitoneal and mesenteric lymph nodes seen with the largest measuring up to 3.6 × 2.0 cm (Fig. 1). Acid-fast bacillus (AFB) blood cultures were ordered due to concerns about MAI. The AFB resulted positive for MAI, with susceptibility tests showing resistance to clarithromycin and sensitivity to amikacin (Table 1).
Figure 1. CT of abdomen and pelvis showing multiple enlarged retroperitoneal and mesenteric lymph nodes, seen with the largest measuring up to 3.6 × 2.0 cm
Table 1. Susceptibility pattern of the Mycobacterium avium-intracellulare complex
The patient refused to go to the hospital because he was enrolled in a hospice and requested outpatient treatment. He was started on once-daily intravenous amikacin via a peripherally inserted central catheter line, in addition to rifabutin, ethambutol and moxifloxacin by mouth. The patient had an excellent clinical response, with a reduction in his abdominal distension and pain. After eight weeks, amikacin was discontinued. The patient was compliant, per his report, with rifabutin, ethambutol and moxifloxacin. He still reported some fever and sweats. Follow-up AFB blood cultures two months into treatment initiation were still positive for MAI, raising concern again regarding compliance. His liver function tests normalised. However, due to increasing values of HBV DNA (750 million IU/ml) despite reported compliance with treatment, hepatitis B virus drug resistance testing was ordered, which showed no resistance to lamivudine/emtricitabine. Tenofovir susceptibility could not be reported. He is currently four months into his MAI and HBV therapy with ongoing fevers, weight loss and abdominal distension. He continues with monthly CAB/RPV LA injections. His CD4 count is 23 cells/mm3, and he remains in the hospice.
DISCUSSION
Management simplification has been the goal of recent HIV drug research to improve patient adherence, decrease the adverse effects of medications and improve the overall quality of life[1]. The combination regimen of two long-acting injectable drugs cabotegravir and rilpivirine, an integrase strand transfer inhibitor (INSTI) and a non-nucleotide reverse transcriptase inhibitor (NNRTI) respectively, have been studied in two clinical trials – ATLAS and FLAIR[1,2]. The findings of these two trials built the foundation for the ATLAS-2M non-inferiority study comparing the once-monthly CAB/RPV regimen to the every-two-month regimen[3].
The approved indication for CAB/RPV LA is an ART switch in a virologically suppressed patient to maintain this suppression. Because of this patient’s likely progression to death in the short term without effective ART, he was initiated on CAB/RPV LA despite his viral load being 300,000 copies/ml. We chose a once-a-month regimen to ensure close follow-up given his history of non-compliance and to test quickly for any emerging resistance should he fail to suppress. In this patient, the initiation of this regimen led to rapid virological suppression within two months, which has been maintained now for six months. Luckily, he appears not to have been harbouring any significant archived INSTI or NNRTI resistance which could have led to CAB/RPV failure. The data on CAB/RPV LA use in non-virologically suppressed patients is slim. Ward 86, an HIV clinic based at San Francisco General Hospital/University of California, is studying and offering long-acting injectable ART for HIV treatment to patients who have challenges with oral medication adherence and are not virologically suppressed. In a first demonstration study of 15 patients who initiated CAB/RPV injections between June 2021 and April 2022 with detectable viremia, twelve patients (80%–95%, confidence interval 55%–93%) achieved viral suppression. The other three had a two-log viral load decline by a median of 22 days[6]. Notably, our patient had never achieved viral suppression prior to initiating the CAB/RPV LA, and he achieved it very rapidly. Achieving viral suppression in those who have never been suppressed spotlights the important role these medications could play in those with challenges adhering to oral ART, provided they are not resistant to the given agents. One study showed that 40% of patients with HIV-1 across the United States are less than 80% adherent to their medication regimen[4]; it is important to consider long-acting medications as a solution. Another meta-analysis showed individual barriers to ART adherence vary, including forgetting to take the medications, being away from home, daily routine change, depression, alcohol/substance use, feeling sick, secrecy/stigma, health service-related barriers – including distance to the clinic – and running out of medications[5], which lead to non-adherence. Many of these barriers are solved with intermittent long-acting injectable drugs.
This patient developed reactivation of HBV and disseminated MAI infection due to IRIS, a phenomenon believed to be due to an inflammatory flare from rapid reconstitution and improvement in immunity in the setting of clinically silent microbial antigens or infections[7]. IRIS can cause a paradoxical worsening of a patient’s health status with fever, night sweats and inflammation despite new-found control of the HIV virus. The pathophysiology of IRIS is thought to be linked to an increase in the CD4 T helper and CD8 T-suppressor cells cell count with a reduction in T regulatory cells[7]. Known or reported risk factors for IRIS include an initial low CD4 count, disseminated latent infections prior to initiation of therapy and rapid reduction in HIV viral load. This is considered to be a price that might have to be paid for successful therapy and is difficult to avoid in instances when patients have had low CD4 counts for years, and have not taken prophylaxis well. ART is typically continued if possible, and a search for occult infection is undertaken. The diagnosis of MAI and HBV reactivation in this patient created new challenges since treatment of most opportunistic infections requires good compliance with multiple medications for a prolonged period of time.
This case highlights the efficacy of monthly CAB/RPV LA in rapidly reducing the HIV viral load level in a poorly controlled patient who lacked significant resistance to the two drugs. This is the first known case of IRIS reported in the literature while using CAB/RPV LA. IRIS in the setting of occult MAI is well recognised. It would have likely occurred with good adherence to any regimen which rapidly suppressed the viral load and is unlikely to be due specifically to CAB/RPV. However, CAB/RPV has no activity against HBV, which may have contributed to its reactivation. The patient had serologic evidence of resolution of prior HBV. However, some patients have covalently closed circular DNA (cccDNA) that may remain in hepatocyte nuclei in the long term[8,9]. This patient was monitored closely for an HBV flare and therapy was promptly initiated upon recognition. Further study is needed to better characterise the proper patient selection, dosing interval, monitoring and adverse effects of LA CAB/RPV in those with elevated viral loads.