ABSTRACT
Introduction: Visual seizure is one of the rare complications of poorly controlled chronic hyperglycaemia. This condition can be debilitating for patients. Early recognition and careful control of hyperglycaemia is vital.
Case description: A middle-aged female was found collapsed at her home after missing insulin for several days. She was found to have diabetes ketoacidosis (DKA) and she was started on treatment for DKA. She reported visual hallucinations in the right side of her visual field for a week. Further assessment with EEG and brain MRI suggested an occipital seizure consistent with metabolic disturbances. She was initially started on antiepileptic medication. After strict diabetes control, her symptoms resolved, and she no longer needed antiepileptic treatment.
Conclusion: Experiencing diabetes-related seizures can be terrifying both for patients and their family. Early recognition and quick control of hyperglycaemia is important in treating these patients.
LEARNING POINTS
- Hyperglycaemia can present with different symptoms of osmotic imbalance including seizures.
- All patients presenting with visual seizures should be investigates for all metabolic abnormalities including hyperglycaemia.
- Correction of hyperglycaemia can improve clinical symptoms as well as physical and psychological well-being of patients.
KEYWORDS
Diabetes, Hyperglycaemia, seizure
CASE DESCRIPTION
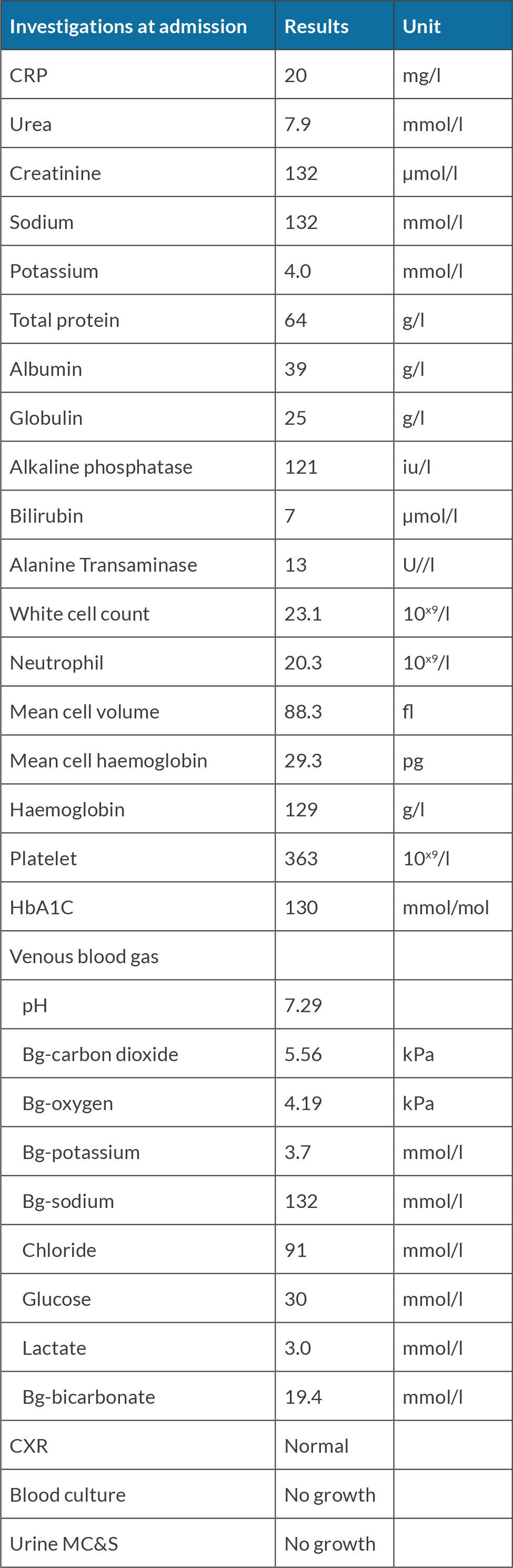
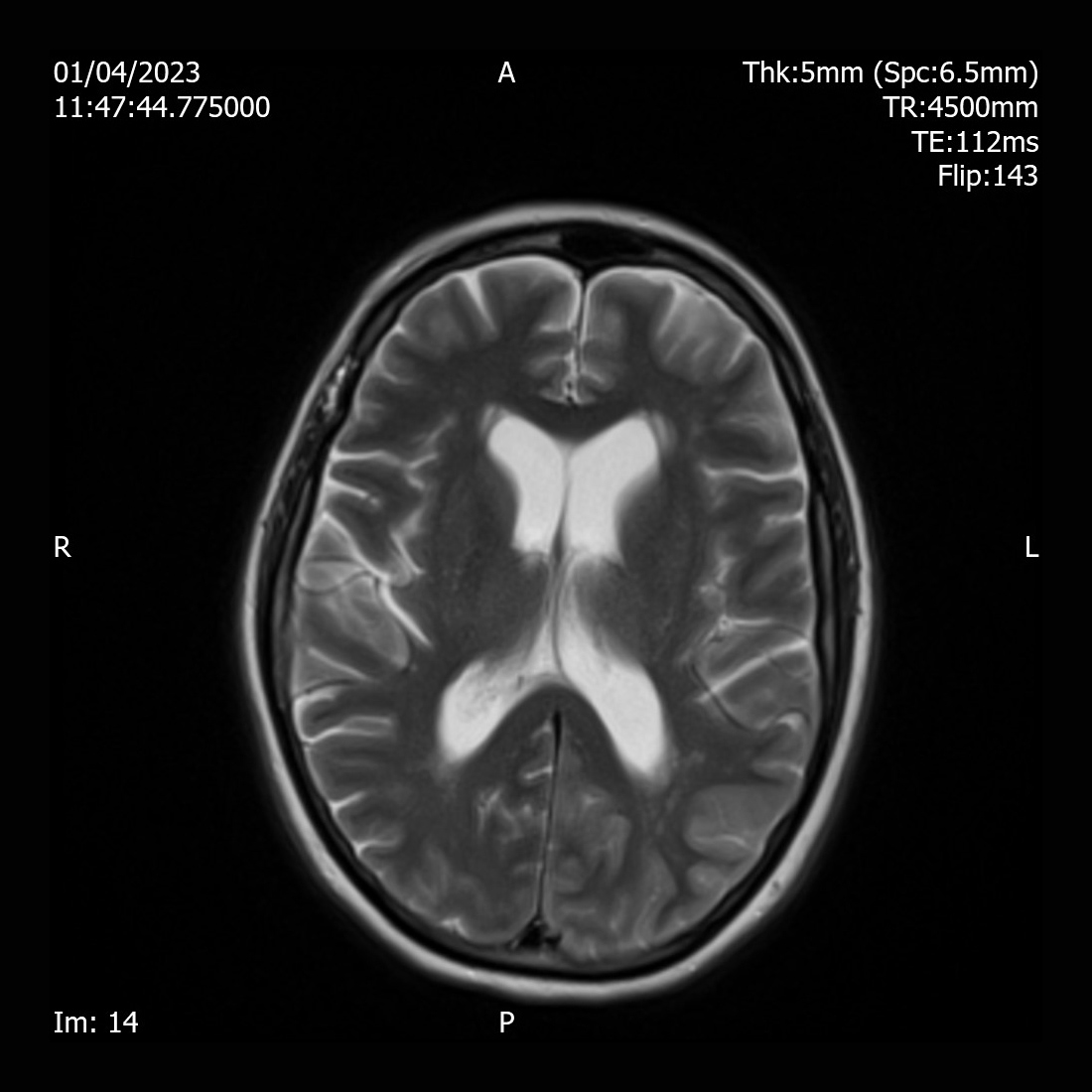
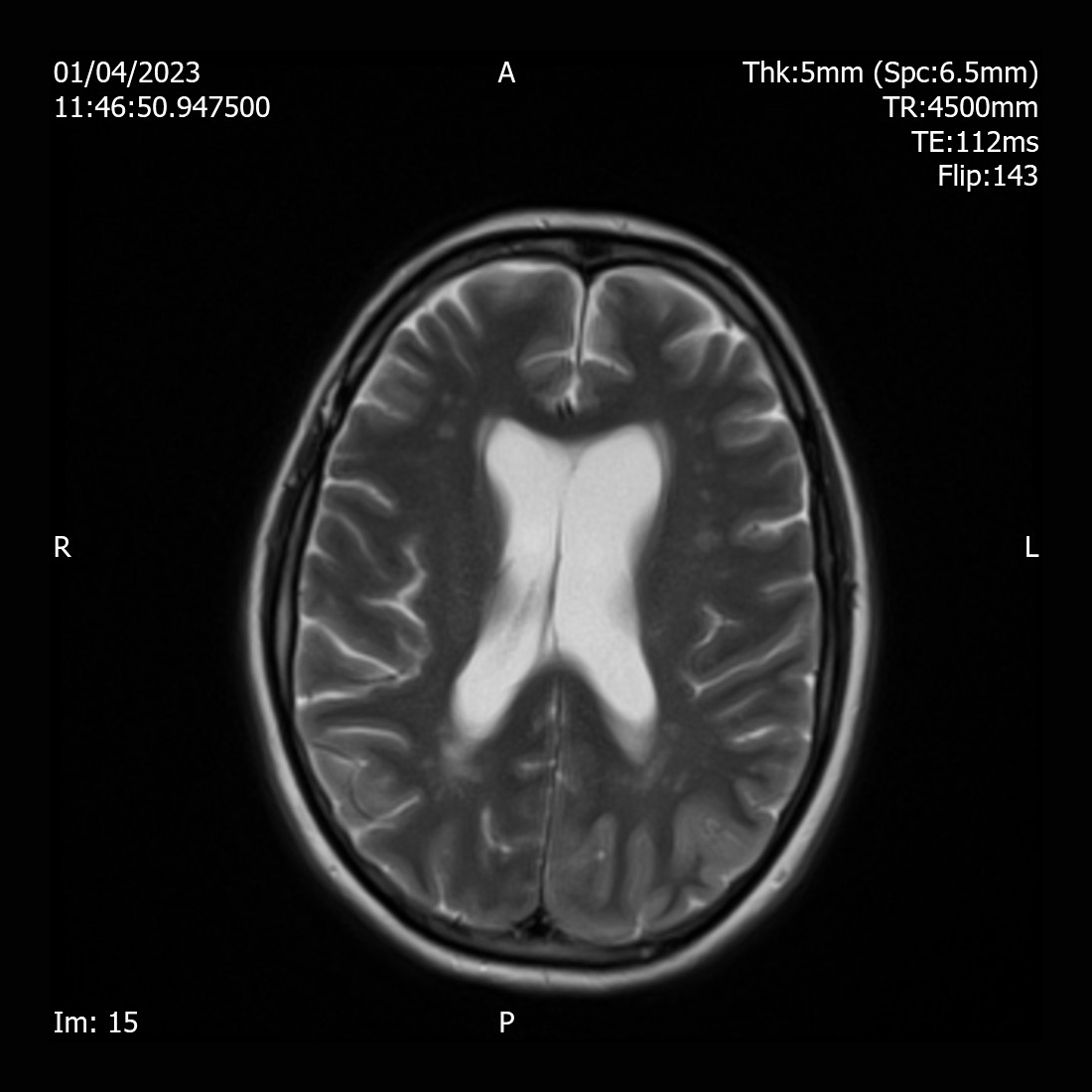
A 55-year-old female was found collapsed at her home and was brought by ambulance to the Emergency Department. She was diagnosed with type 2 dabetes for 20 years and was on metformin 1 g twice daily, gliclazide 160 mg once daily and Abasaglar 42 units once daily. She was started on insulin 2 years ago. Her daughter reported that the patient was found acutely confused. She had diarrhoea and vomiting for days and stopped taking insulin for several days as she felt unwell. She reported that her mother was not compliant with regular insulin injections due to needle phobia. She was not engaging with the community diabetes team. On examination, she was confused and agitated. She had a Glasgow Coma Scale (GCS) of 14/15, tachycardia with a heart rate of 113 bpm, blood pressure of 135/77 mmHg, respiratory rate 16/min and oxygen saturation of 97% on air. She was found to have diabetes ketoacidosis (DKA) with blood glucose of 30 mmol/l, ketone of 7.3 mmol/l and pH of 7.29. Further investigations revealed white cell count 20.3 10x9/l, C-reactive protein (CRP) 20 mg/l, serum sodium 132 mmol/l, potassium 4.0 mmol/l, creatinine 132 µmol/l, haemoglobin A1c ( HbA1c) of 130 mmol/mol (Table 1). She was afebrile and no other signs of infection were identified. She received treatment according to DKA protocol with intravenous fluids and fixed rate insulin perfusion. She improved biochemically and clinically after treatment. Once she recovered cognitively, she complained of significant visual hallucinations significantly affecting her day-to-day life. She reported seeing a streak in her right eye a week previously. The symptom worsened and she eventually had episodes of visual hallucinations which included an individual or group of people playing, or her daughter taking a cup of coffee and pouring it over her. Each episode lasted between 15-20 minutes, and she saw those hallucinations on her right eye only. Her visual field and acuity tests were normal. All the other neurological examination (i.e., cranial nerves, motor, sensory, cerebellar, gait, reflexes, and cognition) were normal. Her EEG showed rhythmic sharp waves in the left occipital region. Brain MRI revealed diffused T2 hyperintensity of the left cerebral cortex involving the left occipital lobe, left posterolateral temporal lobe (Fig. 1), and left parietal lobe (Fig. 2).
Figure 1. MRI T2 showing gyri form diffusion restriction and hyperintensity of the cortex of left temporal lobe
Figure 2. MRI T2 showing gyri form diffusion restriction and hyperintensity of the cortex of left occipital lobe, posterolateral left temporal lobe and left parietal lobe
She was started on levetiracetam 500 mg twice a day and her long-acting glargine insulin dose was titrated up from 24 to 50 unit once a day. The patient was discharged from hospital with referral to the district nurse to observe insulin injections with the aim to improve compliance. Her blood glucose concentration improved thereafter and her visual hallucinations resolved one week after discharged. She no longer needed antiepileptic medication a week after discharge and had good diabetes control and resolution of visual hallucinations. She is now self-injecting insulin with good compliance as she recognized that her episode of severe DKA resulted from not taking insulin regularly. She no longer had visual hallucinations a week after discharge from hospital and is now enjoying her day-to-day life.
DISCUSSION
Seizures happen when there is too much electrical activity in groups of neurons in the brain[1]. Most of the times, seizures end within a few minutes and people recover quickly. Frequent seizures can however severely limit a person’s quality of life, including incapacity from driving and requiring regular supervision of day-to-day activities[2] There are several causes of visual seizures including diabetes. Hypoglycaemia is one of the most common causes of seizures and other neurological presentations in clinical practice with diabetes. However, high blood glucose can also lead to seizures, even status epilepticus without awareness[3]. The mechanism by which neurological dysfunction occurs is yet unclear. A prevailing theory is that in the setting of profound hyperglycaemia, the Krebs cycle is inhibited leading to depletion of gamma-aminobutyric acid (GABA) levels via increased metabolism, thereby lowering the seizure threshold. Other explanations of seizures include osmotic diuresis and dehydration, as well as hyponatremia, and hyperglycaemic damage to cerebral vasculature. Prolonged exposure to hyperglycaemia is the most important risk factor for seizure in those patients. Improvements of hyperglycaemia and osmolar status usually improves neurological manifestations. In conclusion, it is important to recognise the role of good hyperglycaemic control in those patients and early recognition and treatment of hyperglycaemia usually resolves the symptoms.