ABSTRACT
According to the modified World Health Organization (WHO) classification, pregnant women with mechanical valves face a very high risk of complications (Risk Category III). Mechanical valve thrombosis is a serious complication that significantly increases during pregnancy due to multiple mechanisms. Thrombolytic therapy has recently been used as a first-line treatment for mechanical valve thrombosis during pregnancy. However, the consensus regarding the optimal treatment strategy, type, dose and route of administration was unclear. We present three cases of mechanical mitral valve thrombosis during pregnancy treated successfully with repeated doses of ultraslow infusion of low-dose tissue-type plasminogen activator (t-PA) alteplase. We also present a review of the literature on this subject.
LEARNING POINTS
- Pregnancy in women with mechanical heart valves significantly increases the risk of maternal mortality or severe morbidity.
- Non-compliance with anticoagulant therapy and/or less frequent monitoring of therapeutic levels during pregnancy can result in serious complications such as valve thrombosis and thromboembolism.
- Thrombolytic therapy with low-dose tissue-type plasminogen activator can be an attractive alternative to surgical valve replacement and medical treatment in appropriately selected pregnant women with thrombosis of a mechanical valve.
KEYWORDS
Pregnancy, mechanical valve, thrombosis, thrombolytic therapy
INTRODUCTION
According to the 2020 American College of Cardiology/American Heart Association (ACC/AHA) Guideline and the 2018 European Heart Association Guideline, under Class I recommendations, pregnant women with a mechanical prosthesis should be monitored in a tertiary care centre with a dedicated multidisciplinary team of cardiologists, surgeons, anaesthesiologists and maternal-fetal medicine obstetricians with expertise in the management of high-risk cardiac conditions during pregnancy. Anticoagulation is considered mandatory to prevent thrombosis of the mechanical prosthesis. Thus, throughout pregnancy, all women with mechanical valves should receive uninterrupted anticoagulation at a therapeutic level suitable for the type and position of the valve. For a successful pregnancy outcome, strict compliance is crucial for each pregnant woman. Unfortunately, none of the anticoagulation strategies is optimally safe for the mother and fetus. Non-compliance with anticoagulant therapy and/or less frequent monitoring of therapeutic levels during pregnancy can result in serious complications such as valve thrombosis and thromboembolism. Treatment includes optimizing anticoagulation intravenously with the resumption of oral anticoagulation in non-critically ill patients with recent subtherapeutic anticoagulation, and surgery when anticoagulation fails and in critically ill patients with obstructive thrombosis. Given the high fetal loss rates with cardiac surgery during pregnancy, thrombolysis is an attractive alternative for appropriately selected, haemodynamically stable women with mechanical valve thrombosis. We report three cases of pregnant women who experienced thrombosis of a mitral mechanical valve and were treated successfully using a low-dose ultraslow infusion of alteplase.
CASE 1
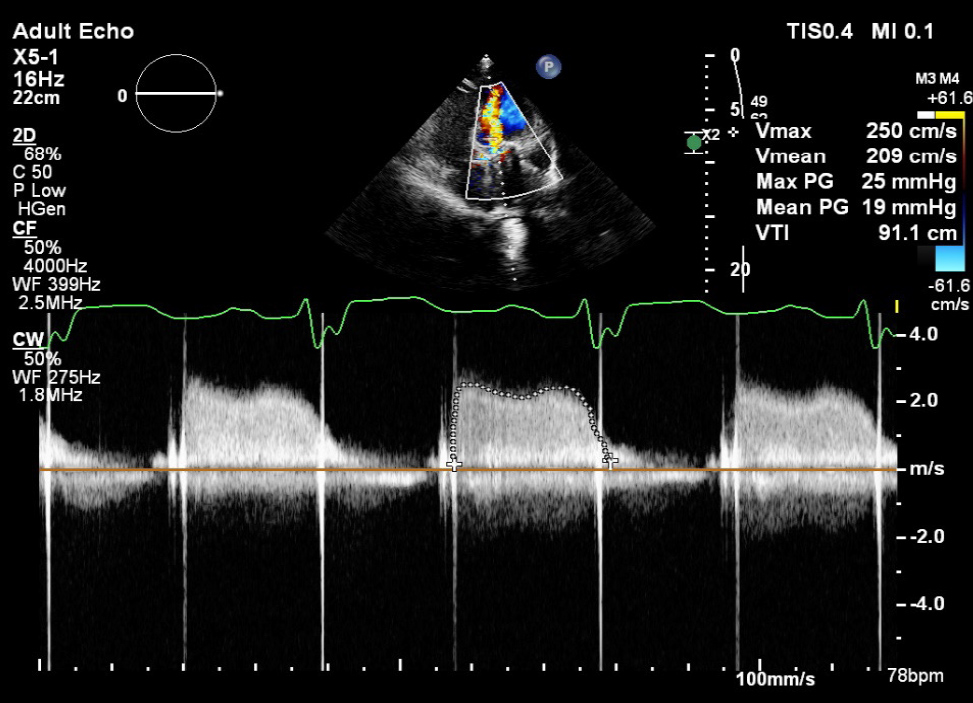
A 23-year-old woman presented at 14 weeks of pregnancy (gravida 1, para 0) with a history of congenital heart disease and atrioventricular septal defect, which had been treated at age 11 with closure of the ventricular and atrial septal defect in addition to mechanical mitral valve replacement (MVR). The patient presented to the cardiac-pregnancy clinic complaining of mild shortness of breath, New York Heart Association II (NYHA II). Since she found out she was pregnant, she had been taking subcutaneous (SC) unfractionated heparin (UFH) 5000 units twice daily for unclear reasons. On clinical examination, her vital signs showed a blood pressure of 100/60 mmHg and a heart rate of 85 beats/min, and on auscultation, heart sounds were minimally heard. Her international normalized ratio (INR) was 1.3. Because of the recent symptoms with inappropriate treatment, transesophageal echocardiography (TEE) was performed and revealed a mechanical bileaflet prosthesis in the mitral position with normal lateral occluder motion; however, the medial occluder was fixed due to a thrombus measuring 0.7 cm2 (Fig. 1). Colour flow Doppler across the prosthesis showed accelerated flow (Fig. 2). The Doppler parameters across the prosthesis were calculated and showed the following values: a mean pressure gradient (MPG) of 19 mmHg at a heart rate of 78 beats/min (Fig. 3), a peak early filling velocity (E wave) of 2.4 m/sec, and a pressure half time (PHT) of 354 ms. The TEE images and Doppler parameters were indicative of metallic valve thrombosis with obstruction. The patient was admitted and started on intravenous (IV) UFH, and the decision was to begin thrombolytic therapy since she had mild symptoms and was haemodynamically stable. The ultraslow infusion of low-dose alteplase (t-PA) protocol was started: 25 mg alteplase over 25 hours of infusion without bolus administration. Doppler transthoracic echocardiography (TTE) was performed every 12 hours after the start of the t-PA infusion. After completion of the first dose, the follow-up Doppler TTE showed a persistent obstructive pattern. The second dose of 25 mg t-PA was started for the next 25 hours. Between the two sessions of t-PA, an infusion of IV UFH was administered with a target-activated partial thromboplastin time (APTT) of 60–80 ms. UFH was withheld during the t-PA infusion. The follow-up Doppler TTE after the end of the second dose of t-PA showed normalization of the mean gradient and E wave value, which indicated relief of obstruction. TEE confirmed the normal motion of both occluders and the absence of the thrombus mass. Immediate infusion of UFH was started in addition to warfarin. When the INR reached 3, the UFH infusion was stopped. The total dose of t-PA used was 50 mg. The patient was discharged on a warfarin dose of 5 and 7.5 mg every other day to maintain an INR of 3.
Figure 1. Transesophageal echocardiography showing a fixed medial occluder of mechanical mitral valve due to thrombus (red arrow). LA: left atrium; LV: left ventricle
Figure 2. Transesophageal echocardiography showing a color Doppler accelerated flow through a mechanical mitral valve related to a fixed medial occluder (red arrow) denoting obstructive pattern. LA: left atrium; LV: left ventricle
Figure 3. Continues Doppler flow across mechanical valve showing mean gradient of 19 mmHg at heart rate of 78 beat per minute highly suggestive of obstructive pattern
CASE 2
A 34-year-old woman presented at 17 weeks of pregnancy (gravida 2, para 1) with a history of rheumatic mitral valve disease treated with mechanical MVR 3 years previously. Before becoming pregnant, the patient had been taking 7.5 mg of warfarin every day. She was shifted to low-molecular-weight heparin (enoxaparin) 60 mg SC without measurement of the anti-Xa factor level since the 13th week of pregnancy. She was referred to a cardiac-pregnancy clinic due to progressive shortness of breath, NYHA III. Her vital signs were stable on examination, and no metallic sound was heard on auscultation. Her INR was 1.5. The TEE showed a metallic bileaflet valve in the mitral position with reduced motion of both occluders and two thrombotic masses located on both occluders measuring 0.6 cm2 and 0.4 cm2, respectively. The Doppler parameters across the prosthesis showed evidence of obstruction (MPG of 18 mmHg, E wave of 2.5 m/sec, and PHT of 340 ms). The patient was admitted and started on an infusion of UFH. A protocol of t-PA infusion similar to that of Case 1 was applied in this case, and after the completion of the third dose of t-PA, Doppler echocardiographic parameters showed obstruction relief, which was confirmed on TEE with normal motion of both occluders and only a minimal residual of thrombotic mass of 2 mm. Clinically, the patient’s symptoms improved, with no more shortness of breath. She was started on a UFH infusion and warfarin. The total dose of t-PA used was 75 mg. She was discharged on 5 mg warfarin with an INR of 3.1.
CASE 3
A 28-year-old woman presented at 22 weeks of pregnancy (gravida 4, para 2) with a history of rheumatic mitral valve disease treated with metallic MVR 6 years previously. She was on warfarin 5 mg daily from the beginning of pregnancy but with no regular follow-up or strict control of INR. She presented to the cardiac-pregnancy clinic with increasing shortness of breath, NYHA III. Her vital signs were stable, no metallic sound was heard on auscultation, and her INR was 1.8. TEE showed a metallic bileaflet prosthetic valve with a fixed medial occluder due to a thrombotic mass measuring 0.8bcm2. Doppler parameters showed an obstructive pattern (MPG of 16 mmHg, E wave velocity of 2.3 m/sec, and PHT of 298 m/sec). The patient was admitted to the hospital and started on UFH infusion. Thrombolytic therapy with the t-PA protocol was initiated. After the completion of the fourth session with a total dose of 100 mg of t-PA, the echocardiographic findings revealed resolution of the obstruction pattern and normal movement of both concluders. UFH infusion resumed with warfarin. The patient’s general condition improved and she was discharged home on 7.5 mg of warfarin daily with an INR of 3.2.
DISCUSSION
The World Health Organization (WHO) has classified pregnancy in women with mechanical heart valves as Risk Category III with a significantly increased risk of maternal mortality or severe morbidity. Valve thrombosis and haemorrhagic complications related to the obligatory use of anticoagulation therapy are considered the main risks. As reported in many prospective registries, pregnancy in this group of women is associated with a maternal mortality rate of approximately 1% and a risk of valve thrombosis of 5%[1]. In the Registry OF Pregnancy And Cardiac disease (ROPAC), the event-free pregnancy rates for women with a mechanical valve were only 58% compared with 78% for women with heart disease but no valve prosthesis[2]. Only 28% of pregnant women and babies had a good outcome in this group, according to a recent UK study[3]. Because of this substantial risk of adverse maternal and fetal events, the guidelines of both ACC/AHA 2020 and ESC 2018 under Class I recommendations mentioned that pregnant women with a mechanical prosthesis should be monitored in a tertiary care centre with a dedicated multidisciplinary team with expertise in managing high-risk cardiac conditions during pregnancy[4,5].
Pregnancy is a hypercoagulable process and state mostly due to increased production of factor VII and fibrinogen, which probably helps to decrease the risk of haemorrhage during and after delivery[6]. For this reason, the management of pregnant women with prosthetic heart valves is significantly different from that of non-pregnant patients, as the risk of mechanical valve thrombosis is much higher. With adequate dosing of anticoagulation therapy, the risk of valve thrombosis is lower. However, this depends on the type and position of the mechanical valve and the association with other patient-related risk factors[7]. Of 202 pregnant women with mechanical valves, 4.7% had valve thrombosis, and the mortality rate was 20%, as reported in the ROPAC registry[2].
The anticoagulation strategy during pregnancy with a metallic valve requires specialized expertise and frequent titration of vitamin K antagonists (VKAs) or heparin doses[8]. With VKAs throughout pregnancy, the risk of valve thrombosis is relatively low (0%–4%)[2], whereas the risk is significant (9%–33%) with UFH in the first trimester or throughout pregnancy in addition to the other risks of thrombocytopenia and osteoporosis[9]. Low-molecular-weight heparin (LMWH) throughout pregnancy with anti-Xa monitoring and dose adjustment according to peak levels carries a valve thrombosis risk of 4.4%–8.7%[9]. Each anticoagulation strategy has relative advantages and disadvantages regarding maternal and fetal safety. The risks to the mother and fetus should be carefully balanced when choosing the appropriate anticoagulation strategy, and this requires a team familiar with the management of prosthetic heart valves in pregnancy to provide comprehensive counselling. No anticoagulation strategy is consistently safe for the mother and fetus. Despite the lack of adequate randomized studies, the use of VKAs throughout pregnancy in this category of women under strict INR control is considered the safest strategy to avoid thrombosis of the mechanical valve[10]. Strict compliance is crucial for good outcomes, whatever the chosen anticoagulation strategy, and the mother should understand this. The most effective approach to prevent thrombosis of the mechanical valve is the use of VKAs (warfarin) because of the low risks of embryopathy, fetopathy (<2%) and fetal loss (<20)[10]. Warfarin has a dose-dependent teratogenic effect where a dose equal to or less than 5 mg/day is associated with a reduced risk of embryopathy but does not totally eliminate it[11]. When the dose of warfarin required for the pregnant woman is equal to or <5 mg/day, the continuation of low-dose warfarin throughout pregnancy poses the lowest combined risk to the mother and fetus[11]. If the required dose is >5 mg/day during the first trimester, the risk of fetal loss or embryopathy is >30%. In this case, replacing warfarin with dose-adjusted LWMH during the first trimester reduces fetal loss[12]. One of the crucial steps while a pregnant woman is on LMWH is monitoring the anti-Xa levels at least weekly with adjustment of the dose accordingly. It is never appropriate to use fixed dosing of LMWH because it is associated with high maternal morbidity and mortality[13]. The teratogenic effect of warfarin after the first trimester drops significantly. Switching back to warfarin is reasonable in the second and third trimesters to balance maternal and fetal safety. At 36 weeks, the best strategy is to shift the pregnant woman to parenteral heparin (UFH IV with APTT control or LWMH SC twice daily). Moreover, UFH IV with APTT control more than or equal to twice control should be established 36 hours before a planned delivery. UFH should be stopped 4–6 hours before delivery and restarted 4–6 hours after delivery if there is no bleeding.
For the mother and fetus, prosthetic valve thrombosis (PVT) during pregnancy is life-threatening. Progressive shortness of breath and/or an embolic event are reasons for immediate TTE to search for valve thrombosis, usually followed by TEE. Management of valve thrombosis is comparable with management in non-pregnant patients[4]. Cardiac surgery in pregnancy is associated with very high maternal and fetal mortality (6% and 30%, respectively) and morbidity (24% and 9%, respectively)[14]. Although thrombolytic therapy during pregnancy is considered a relative contraindication[15], thrombolytic therapy with t-PA can be an attractive alternative to surgical valve replacement in appropriately selected pregnant women with thrombosis of a mechanical valve. The suggested optimal candidates are pregnant patients with an obstructive prosthetic valve and stable haemodynamics, pregnant patients presenting with an embolic complication with valve thrombosis, or pregnant patients with non-obstructive valve thrombosis with a thrombus >10 mm[15]. In 2013, Ozkan et al. evaluated the safety and efficacy of a low-dose (25 mg), slow infusion (6 hours) of t-PA for the treatment of PVT in pregnant women where 28 PVT episodes (15 obstructive and 13 non-obstructive) were treated with a low-dose, slow infusion of t-PA. They concluded that the low-dose, slow infusion of t-PA with repeated doses as needed is an effective therapy with an excellent thrombolytic success rate and seems to be safer than cardiac surgery or any alternative medical strategies[16]. In this study, there were 20 live births and five miscarriages (20%), while none of the pregnant patients developed systemic thromboembolism after thrombolytic therapy and there was no maternal mortality. They also reported that the maternal and fetal adverse events were lower than in those with surgery or medical therapy. Because of concern about an increased risk of spontaneous abortion with thrombolytic therapy in pregnancy, in 2015, Ozkan et al. published the Ultra-slow PROMETEE trial where they tried to evaluate if further prolongation of the infusion time may reduce the complication rates without reducing success rates[17]. For all patients admitted with PVT (120 episodes with 64.2% obstructive and 35.8% non-obstructive), TEE-guided thrombolytic therapy was given as an ultraslow infusion (25 hours) of low-dose (25 mg) t-PA. The protocol included 25-hour infusion of 25 mg t-PA without bolus to all included patients (patients with obstructive thrombus, non-obstructive thrombus either with or without a history of recent thromboembolism, and a thrombus diameter of ≥10 mm). Intermittent Doppler TTE was performed in patients with obstructive valves every 12 hours after the start of t-PA infusion. If TTE showed haemodynamic normalization of the prosthesis, the t-PA infusion was interrupted and immediate TEE was performed to confirm the normalization. If complete success was confirmed by TEE, then no more thrombolytic therapy was given and UFH was initiated. In case of partial improvement, the thrombolytic therapy continued with TTE follow-up every 12 hours. If needed, thrombolytic therapy was repeated up to eight times with a total dose of 200 mg. Between the completed sessions of infusion, UFH was administered for 6 hours. The Ultra-slow PROMETEE trial showed that the ultraslow strategy seems to be safer than the slow strategy in terms of complication rates (6.9% vs. 10.5%, respectively), while the overall success rate was 90%[17]. Recently, thrombolytic therapy has been used as a first-line treatment in this category of patients[18]; however, the consensus concerning the optimal strategy of treatment, type, dose and route of administration was not clear.
In our case series, the use of an ultraslow infusion and low-dose protocol of t-PA administration in pregnant women with mitral mechanical valve thrombosis showed excellent results with no complications.
CONCLUSION
Thrombolytic therapy with t-PA can be an attractive alternative to surgical valve replacement and medical treatment in appropriately selected pregnant patients with thrombosis of mechanical valves with a high level of safety.