ABSTRACT
Overall gastric cancer incidence is decreasing, but incidence of gastric signet ring cell carcinoma has been rising. The diagnosis can be challenging. It has a poorer prognosis because it tends to be diagnosed at advanced stages. Lymphedema is a rare presentation. We report a rare presentation of signet ring cell carcinoma in a 49-year old male, with no underlying medical condition. The patient presented with lymphedema of lower limbs, scrotum and abdominal wall.
LEARNING POINTS
- Signet ring cell carcinoma tends to have an infiltrative behavior. Endoscopic analysis may not lead to any macroscopic finding.
- In highly suspicious cases, endoscopic exploration should be complemented with an endoscopic ultrasound or blind random biopsies.
KEYWORDS
Signet ring cell carcinoma, gastric cancer, paraneoplastic syndrome
INTRODUCTION
Despite the decrease in overall gastric cancer incidence, signet ring cell carcinoma incidence has been slowly rising [1]. In contrast with other gastric cancers, the incidence of signet ring cell carcinoma is higher in females and younger patients[1,2]. Signet ring cell carcinoma may share typical symptoms with gastric neoplasms, such as abdominal pain, bloating or weight loss, or it may present with nonspecific paraneoplastic manifestations. Signet ring cell carcinoma is associated with more advanced forms of the disease at diagnosis[1,2]. The stage at diagnosis is believed to be the main prognostic variable[1].
CASE DESCRIPTION
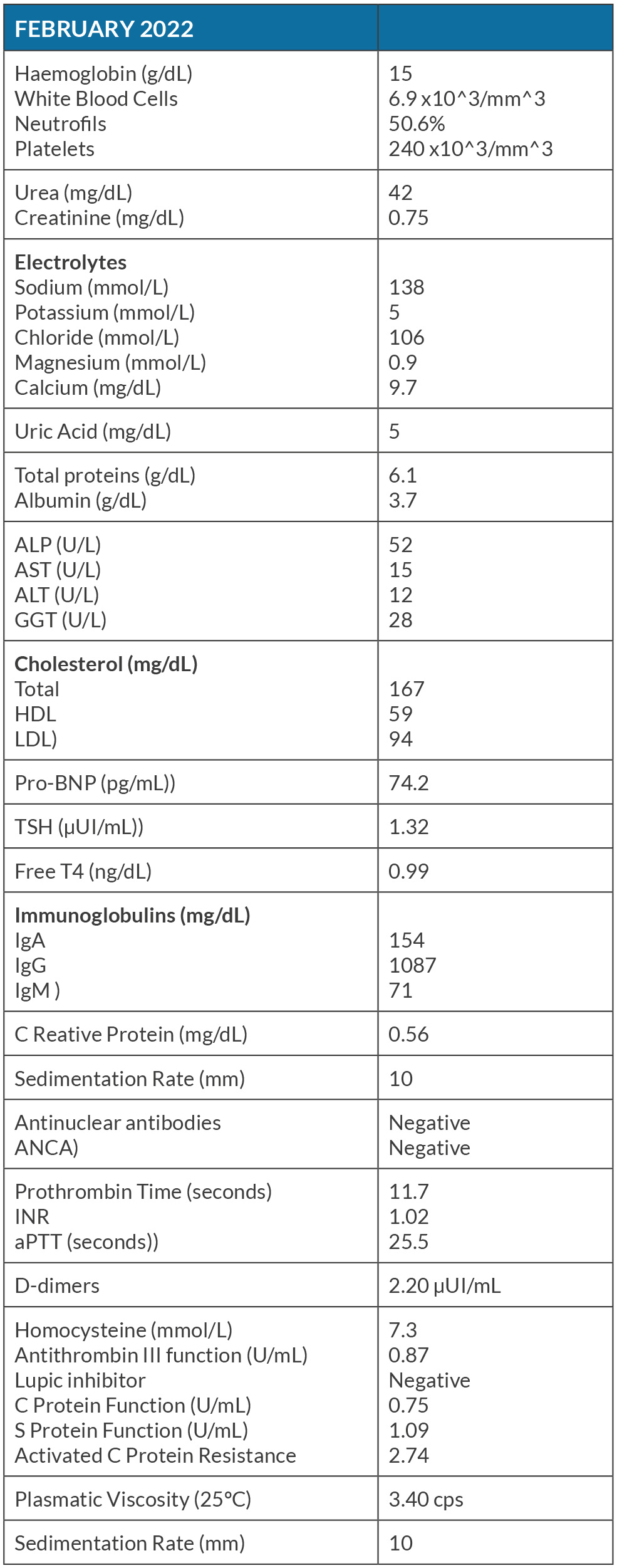
The patient was a 49-year old male, nonsmoker, with an average alcohol consumption of 10 grams a week. His medical history and family history were unremarkable, without known history of cancer. He was referred by his general practitioner because of limb edema which began inexplicably two months prior to the GP consultation and progressively worsened. Deep vein thrombosis was excluded by Doppler-ultrasound. An asymmetrical indurated lymphedema of the limbs with positive Stemmer sign was identified. He did not report any gastrointestinal symptoms. Two months later, the patient’s condition worsened. The lymphedema ascended to the limbs, scrotum and abdominal wall. A nonspecific adipose and subcutaneous tissue densification of hypogastric regions and of pelvis was the only finding in his CT. Laboratory analyses were normal, except for elevated D-dimers (Table 1).
Table 1. Laboratory analysis (in bold are highlighted the altered results).
ALT - Alanine transaminase; ALP - Alkaline Phosphatase; ANCA - Antineutrophil Cytoplasmic Antibodies; aPTT - Activated Partial Thromboplastin Time; AST - Aspartate aminotransferase; GGT - Gamma-glutamyl tansferase.
Autoimmune and prothrombotic tests were negative. He was sent to the emergency department. A CT angiography was performed and no signs of thrombotic events or compression of structures were found. Nonetheless, imaging revealed small paratracheal, aortic and perihilar adenopathies and lesser gastric curvature nonspecific adenopathies, with a dominant adenopathy of 19x15 mm. The patient was admitted for further analyses.
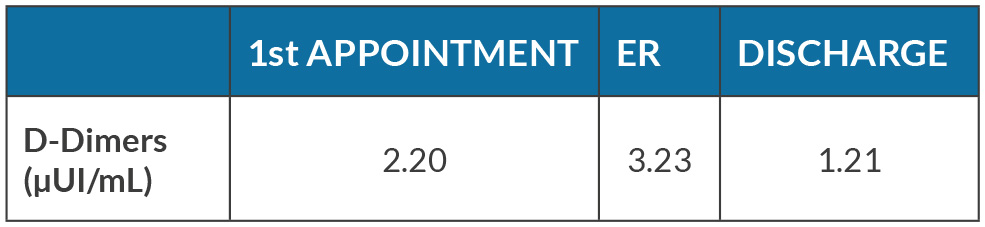
Nephrologic, liver and thyroid diseases were excluded. Heart failure was also excluded and a transthoracic echocardiogram was normal. Doppler examination of limbs and CT angiography of major vessels found no obstruction or thrombus. Because of the dominant perigastric adenopathy he underwent an abdominal MRI and upper gastrointestinal endoscopy that were both normal. To this time, all analyses were negative, except for persistent elevated D-dimers (Table 2) and elevated blood viscosity. Prophylactic anticoagulation with enoxaparin was initiated and maintained during his stay. The treatment was discontinued on discharge.
Table 2. (A, B) Chest CT scan at the presentation shows a soft-tissue attenuation lesion that contains a fatty centre located in the epipericardial fat at the left cardiophrenic angle. (C, D) Follow-up chest CT scan 3 months after presentation reveals disappearance of the soft-tissue lesion.
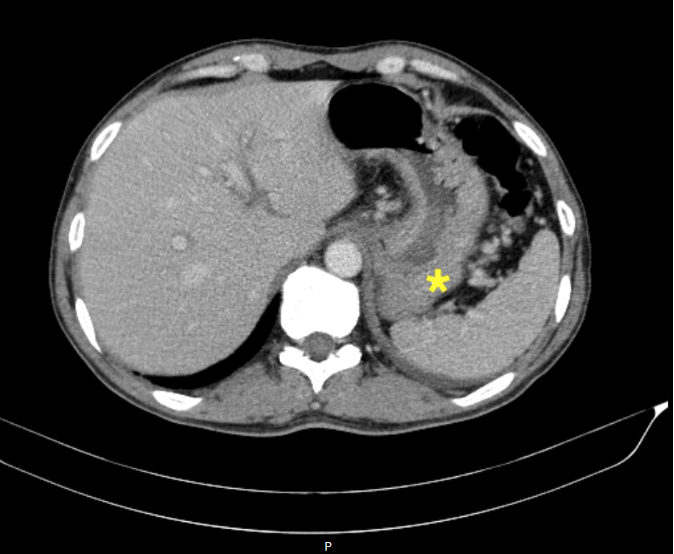
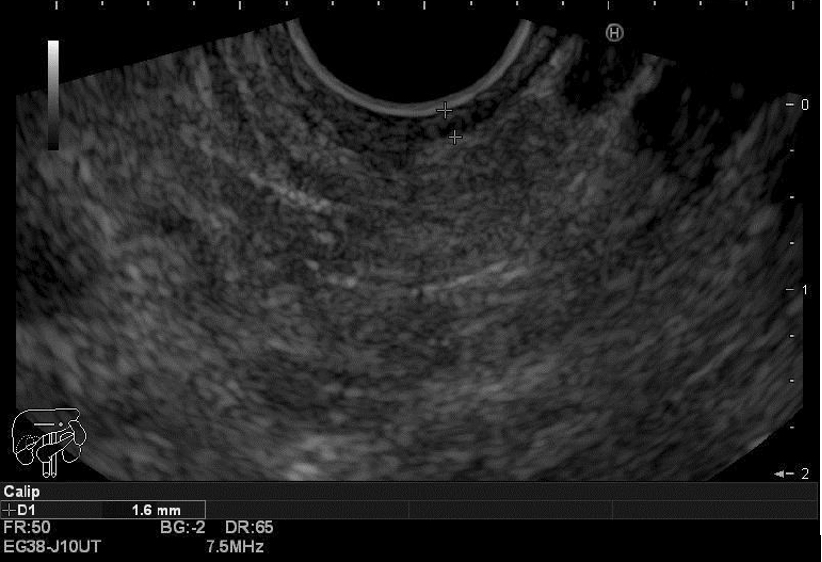
One week later, he underwent further examination in an ambulatory setting, and again a clinical worsening was evident. The lymphedema had worsened and a new painful left cervical tumefaction was evident. A CT angiogram revealed left jugular vein thrombosis and bilateral segmental pulmonary thromboembolism. A pre-carinal adenopathy of 15mm and a discreet and irregular parietal thickening of gastric fundus were evident in the venous phase of contrast injection – elements which were not evident in previous scans (Figure 1). Anticoagulation with enoxaparin was restarted, but in therapeutic dose. The persistent elevation of D-dimers and evidence of thrombotic events were interpreted in the context of a paraneoplastic syndrome. Because of gastric fundus thickening, even though the endoscopy was normal, an endoscopic ultrasound was made, that revealed an infiltrative lesion of 5 mm in depth in the small curvature of the stomach with mucosal, submucosal and muscularis propria involvement (Figure 2), and multiple perigastric adenopathies. These elements are suggestive of gastric neoplasia with ganglion involvement. Multiple tissue samples were taken and biopsies revealed poorly cohesive signet ring cell carcinoma.
Figure 1. Gastric wall thickening (*) evident in the venous phase of the CT contrast injection.
Figure 2. Hypoechoic thickening on endoscopic ultrasound suggestive of an infiltrative lesion.
DISCUSSION
Signet ring cell carcinoma is a specific adenocarcinoma of the stomach[2]. It has a higher rate of regional and disseminated invasion at diagnosis than other gastric cancers[3].
In patients with cancer, lymphedema is usually a complication of treatment such as lymphadenectomy or radiotherapy, or a result of carcinomatous lymphangitis. In this case, there was no mechanical cause nor history of previous treatment, which made cancer the most probable hypothesis. Cutaneous biopsies would be of interest to confirm lymphogenic dissemination.
Because the first endoscopic study showed no macroscopic abnormalities, no biopsy was made at the time of first admission. Signet ring cell carcinoma tends to have an infiltrative behavior, in contrast to other gastric cancers that usually evolve to present ulcerations. In highly suspicious cases it may be of interest to complement observations with an endoscopic ultrasound or, if not available, with blind random biopsies. Additionally, PET-scans are usually associated with lower FDG uptake, which compromises their overall sensitivity and increases the rate of false negatives. This increases the complexity in analyzing findings and hinders the diagnosis approach.
The first time the patient was discharged he had persistently elevated D-dimers and increased blood viscosity, but no documented vascular thromboembolic events. There is no consensus about the timing to start prophylactic anticoagulation in patients with paraneoplastic syndromes and without thromboembolic events while the primary cause is still occult. The authors defend that anticoagulation could be initiated at that moment, but an individual decision must be made for each patient.
While writing this report, we only found two similar cases in which a clinical association between lymphedema and gastric signet ring carcinoma has been made[4].