ABSTRACT
Clostridioides difficile infection (CDI) commonly presents with diarrhoea, colitis, and in more severe cases, toxic megacolon. Extraintestinal Clostridioides difficile infection (EI CDI) is rarely reported. Intraabdominal abscesses are the most commonly reported EI CDI presentation. EI CDI-associated bacteraemia, as well as bone, lung, and even intracranial infections have been reported in the literature. EI CDI is usually seen in patients with multiple comorbidities. Due to the rarity of cases, no clear treatment guidelines exist, but metronidazole and vancomycin have been primarily used to treat EI CDI and additional antibiotics have been used for treatment when the isolates are polymicrobial. We report a case of a patient with significant comorbidities who developed EI CDI following acute ruptured appendicitis. She was successfully treated with drainage of abscess and intravenous metronidazole followed by oral metronidazole.
LEARNING POINTS
- Clostridioides difficile commonly causes colitis and extraintestinal Clostridioides difficile infection is a rare finding.
- Extraintestinal Clostridioides difficile infection occurs due to translocation of flora in the setting of acute inflammation.
- Extraintestinal Clostridioides difficile abscesses are commonly polymicrobial and associated with high mortality.
KEYWORDS
Clostridioides difficile, abscess, intraabdominal, extraintestinal
CASE DESCRIPTION
A 74-year-old female with a past medical history of type 2 diabetes mellitus, stage 3 chronic kidney disease, non-obstructing renal stones, and chronic obstructive pulmonary disease (COPD) with chronic hypoxia was admitted with a chief complaint of constant right lower quadrant abdominal pain, constipation, and nausea that had been ongoing for the previous 3 days. She reported no fever, diarrhoea, dysuria, or blood in the stool. She had a history of appendicitis 6 years prior to this presentation, which was treated conservatively with antibiotics at that time. Her admission vitals were within normal range and examination revealed tenderness to palpation in the right lower abdominal quadrant and periumbilical region.
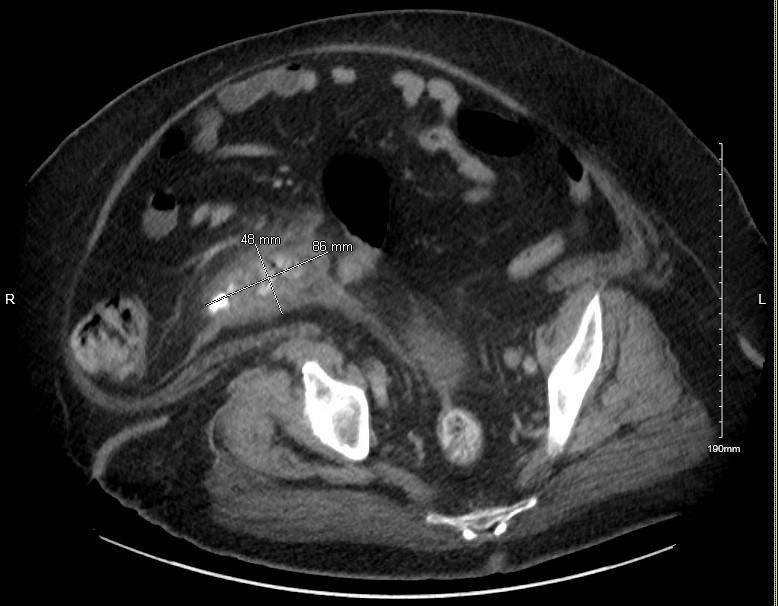
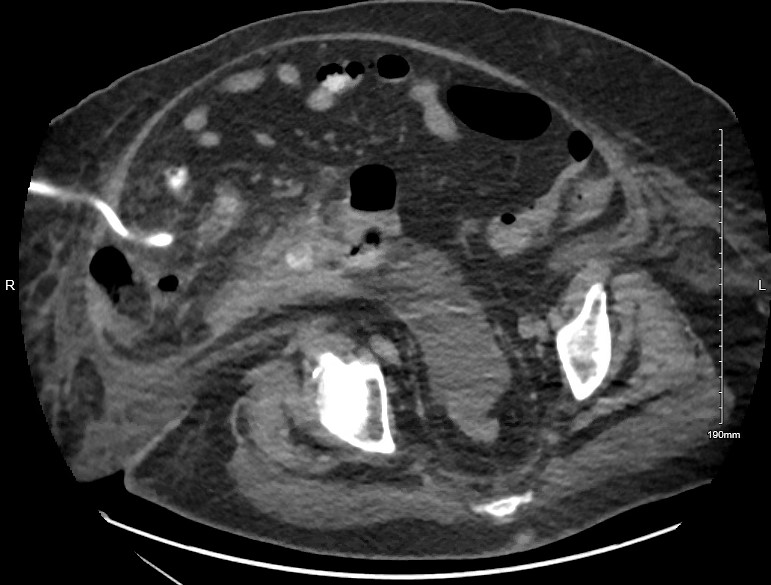
White blood cell count was 13 x 103 with neutrophil predominance. Urine and blood cultures showed no growth. Computed tomography (CT) scan of the abdomen showed a dilated appendix with irregular enhancing walls and periappendiceal stranding consistent with acute appendicitis. A small amount of faecal content and air was seen at the appendiceal tip and calculus was seen at the proximal appendiceal body (Fig. 1) The surgical team recommended conservative management given her comorbidities. Treatment was initiated with intravenous (IV) fluids and piperacillin-tazobactam. She tolerated a clear liquid diet on day 2 of admission and later was advanced to a soft diet. On day 7, the patient complained of diffuse abdominal pain. A repeat CT abdomen showed interval progression of acute perforated appendicitis with an increase in periappendiceal phlegmonous inflammatory changes involving the sigmoid colon and loops of the distal ileum (Fig. 2). The phlegmon was not amenable to drainage by interventional radiology (IR) at this time. Total parenteral nutrition was initiated while maintaining nothing by mouth (NPO) status for bowel rest. A third CT was obtained 12 days after admission, and it showed a complex, thick-walled gas and fluid collection in the lower right abdomen measuring 12.5 x 7.2 x 7.5 cm (Fig. 3). The patient underwent CT-guided aspiration of 115 mL of purulent, malodorous fluid and a pigtail drainage catheter was placed (Fig. 4).
Figure 1. Computed tomography (CT) scan of the abdomen showing features of acute appendicitis with a dilated appendix with irregular enhancing walls and periappendiceal stranding
Figure 2. CT abdomen showing interval progression of acute perforated appendicitis with an increase in periappendiceal phlegmonous inflammatory changes
Figure 3. CT abdomen obtained 12 days after admission showing a complex, thick-walled gas and fluid collection in the lower right abdomen measuring 12.5 x 7.2 x 7.5 cm
Figure 4. CT abdomen with pigtail catheter in place and reduced size of abscess
Microbial cultures from the abscess grew Escherichia coli, Enterococcus avium, and heavy Clostridioides difficile. Ampicillin-sulbactam was initiated to cover E. coli and E. avium and IV metronidazole was added to cover C. diff. The patient started tolerating an oral diet on day 7 of drain placement and parenteral nutrition was discontinued. A repeat CT abdomen pelvis on day 12 of drain placement demonstrated no significant residual collection and the pigtail drain was removed (Fig. 4). IV ampicillin-sulbactam and metronidazole were discontinued and oral metronidazole 500 mg three times daily and amoxicillin 500 mg two times daily were both prescribed at the time of discharge, for a further 7 days . She received a total of 14 days of metronidazole with a good outcome.
DISCUSSION
Clostridioides difficile is a gram-positive, spore-forming, anaerobic bacillus that is estimated to colonize 4-20% of long-term care residents and 2-10% of community individuals [1,2]. The most common form of symptomatic CDI is enterocolitis followed by pseudomembranous colitis, and less commonly, toxic megacolon [3]. Colitis is the most common manifestation of CDI but there are occasional reported cases of extraintestinal infections (EI) including bacteraemia, pyogenic liver abscess, intraabdominal abscess, and brain abscess [4-6]. Overall, the reported frequency of EI CDI is between 0.17% and 0.6% [6]. Most of these EI CDI are either preceded by a gastrointestinal tract infection or a disruption in the anatomic structure of the colon due to surgery or intestinal perforations [2,7] – as in this case where intraabdominal abscess formation was preceded by a ruptured acute appendix.
A single-centre study spanning over 10 years found only 31 cases of EI CDI. Most (85%) of the cases were polymicrobial in nature and 81% of these patients had experienced recent antibiotic exposure. Four out of 31 cases were of intraabdominal infection without prior surgery and three of them were preceded by perforated diverticulitis or appendicitis [3].
The risk factors for EI CDI, identified in the existing literature, include antibiotic exposures, hospitalization, immunocompromised state, advanced age, and gastrointestinal surgery [4,2]. Malignancy, inflammatory bowel disease, liver cirrhosis, and alcoholism have also been identified as other possible risk factors for CDI at unusual sites [8].
The largest retrospective study by Gupta et al. reported 40 cases of EI CDI out of which 25 cases were of intraabdominal infection. A total of 24 the intraabdominal infections were preceded by surgical manipulation of the gut and one case was secondary to acute perforated diverticulitis and pelvic abscess. Most (88%) of cases were associated with prior antibiotic use and 62.5% of the isolates were polymicrobial[7]. Urban et al. investigated the incidence of EI CDI in a tertiary centre in Hungary and 91.6% of isolates were noted to have polymicrobial growth [2]. In another retrospective study by Mattila et al., 85% of cases had polymicrobial growth. In all three studies, most of these patients were found to have prior antibiotic exposure and a high burden of comorbidities [3,7,2].
Our patient had a high Charlson Comorbidity Index score of 8 and did have a history of perforated appendix as a disruption of the gastrointestinal tract. She also received prolonged periods of antibiotics for acute appendicitis before the abscess formation. In our patient, CDI was most likely due to direct translocation from the intestinal lumen via the appendiceal rupture and subsequent seeding in the peritoneal cavity. The direct leakage of CD from the intestines to the abdominal cavity appears to be the most likely mechanism of intraabdominal seeding [8]. The distant sites of EI CDI such as the brain, bones, and lungs are secondary to transient CD bacteraemia [4]. Our patient did not have any diarrhoea, consistent with the study by Mattila et al., where only 51% of identified EI CDI cases reported diarrhoea[3].
There is a rise in the number of reported cases of EI CDI in recent years due to better diagnostic techniques but there might be an underestimation of incidents because C. diff. is a fastidious anaerobe and toxin assays are not commonly used in non-stool samples [7]. There are no established guidelines for treating EI CDI, but the review of available literature supports it being treated in the same way as enteric CDI [2,7]. There is some literature evidence that EI CDI may be due to non-toxin-generating C. diff. Nevertheless, the virulence factors of C. diff. organisms causing EI CDI are not yet clear [9]. Clinically, it is difficult to discern the pathogenic role of the CD when it is found in extraintestinal sites especially when it is a polymicrobial infection.
Treatment with parenteral metronidazole, vancomycin, and piperacillin-tazobactam has been reported in the literature [7]. Other interventions such as drain placement, debridement, and surgical excision may be needed [7], depending on the site of infection. Most patients with EI CDI have multiple comorbidities and a significant number are post-surgery patients. C. diff. antibiotic resistance is a growing concern, but the majority of EI CDI are polymicrobial, thus antibiotic resistance of the other organisms can also determine patient outcomes [10].
CONCLUSION
Clostridioides difficile is a known cause of diarrhoea and colitis but EI infection due to C. diff. is rare. Of note, intraabdominal infections are the most common EI site and most of these infections are polymicrobial. Prolonged hospitalization, immunosuppression, use of broad-spectrum antibiotics, and use of proton pump inhibitors are risk factors for the development of EI CDI and are associated with a high mortality rate.