ABSTRACT
Background: Chronic granulomatous disease (CGD) is a rare immunodeficiency disorder resulting in phagocytic cell dysfunction. It is characterized by deficient cellular immunity against bacteria and fungi, and an excessive inflammatory response resulting in granuloma formation. It manifests, usually in early childhood, with recurrent bacterial and fungal infections or inflammatory complications. The infections, such as invasive pulmonary aspergillosis, can be life-threatening.
Case description: Our patient was a 40-year-old man with no pulmonary history who presented with bilateral pulmonary nodules and pronounced eosinophilia in peripheral blood and bronchoalveolar lavage fluid, mimicking eosinophilic pneumonia. During treatment with corticosteroids, the patient deteriorated clinically and radiographically. Extensive investigations failed to provide a diagnosis. A lung biopsy demonstrated the presence of granulomas and Aspergillus fumigatus hyphae. Advanced screening to detect underlying immunodeficiency revealed CGD.
Discussion: This case report describes a unique first presentation of CGD. It reminds physicians of the possibility of CGD as an underlying immune disorder in invasive aspergillosis and highlights the challenges of diagnosing invasive pulmonary aspergillosis. We discuss the diagnostic pitfalls of this case and propose a diagnostic work-up for eosinophilic lung disease.
LEARNING POINTS
- Pulmonary aspergillosis can present as eosinophilic pneumonia and should be included in the differential diagnosis of eosinophilic lung disease.
- In case of invasive pulmonary aspergillosis, investigation for chronic granulomatous disease should be considered.
- Chronic granulomatous disease in adults is probably underdiagnosed because of its variable clinical presentations.
KEYWORDS
Pulmonary aspergillosis, chronic granulomatous disease, hypereosinophilia, eosinophilic pneumonia, pulmonary infiltrates with eosinophilia
BACKGROUND
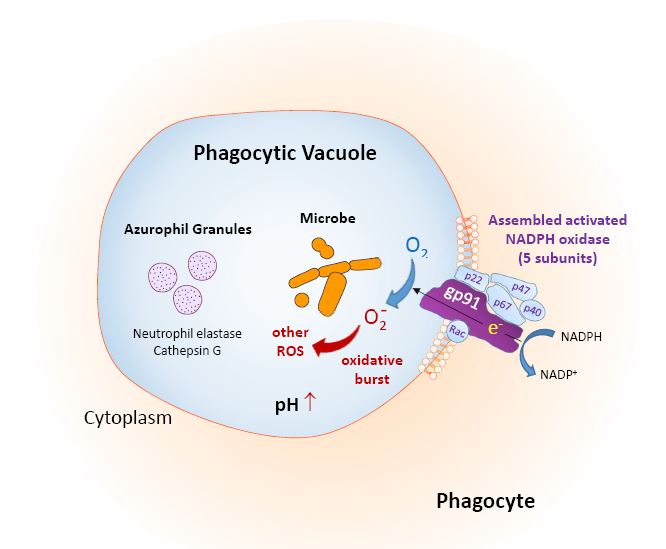
Chronic granulomatous disease (CGD) is an uncommon primary immunodeficiency disorder caused by mutations in the nicotinamide adenine dinucleotide phosphate oxidase (NOX) genes. These mutations result in deficiency of the NOX enzyme. NOX is located in the cell membrane of neutrophils and monocytes. Stimulated by the phagocytosis of microorganisms, it produces superoxide radicals to fight bacteria and fungi, the so-called respiratory burst (Fig. 1). In case of a defective defence mechanism, the persistent phagocyted microorganisms and overstimulation of chemokines leads to the formation of a granuloma [1].
Figure 1. Function of NADPH oxidase in phagocytic cells. Reproduced from: Seger R. Chronic granulomatous disease 2018: advances in pathophysiology and management. LymphoSign Journal 2019;6:1–16.
Patients with CGD present with recurrent bacterial and fungal infections, and inflammatory disorders such as granulomatous lung disease, skin granulomas, inflammatory bowel disease and inflammatory cystitis [1, 2]. The disease is diagnosed by demonstrating the deficient oxidizing activity of granulocytes. Two tests are available: the quantitative dihydrorhodamine 123 (DHR 123) flow cytometry assay and the nitroblue tetrazolium dye test [1].
Management of CGD consists of infection prophylaxis and treatment of complications. The only curative option is stem cell transplantation. Gene therapy is still experimental [1].
Although CGD is a disease of early childhood, we present the case of a middle-aged patient without a relevant medical history and with a unique first presentation of the disease.
CASE DESCRIPTION
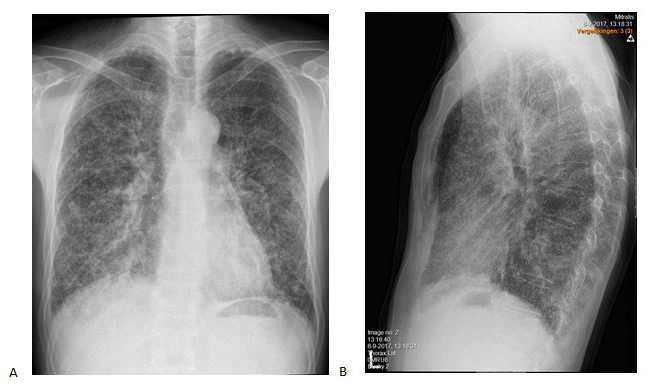
A 40-year-old man was referred to our pulmonology department because of an aberrant chest X-ray (Fig. 2). He complained of exercise-induced dyspnoea and a productive cough. He had a history of Asperger syndrome, recurrent otolaryngological infections and dental abscesses. He had no relevant family history and no toxic exposures. He took no medication. He was an extensive traveller but his last trip was more than 1 year ago.
Figure 2. Chest X-ray at initial presentation: anteroposterior (A) and lateral (B) views
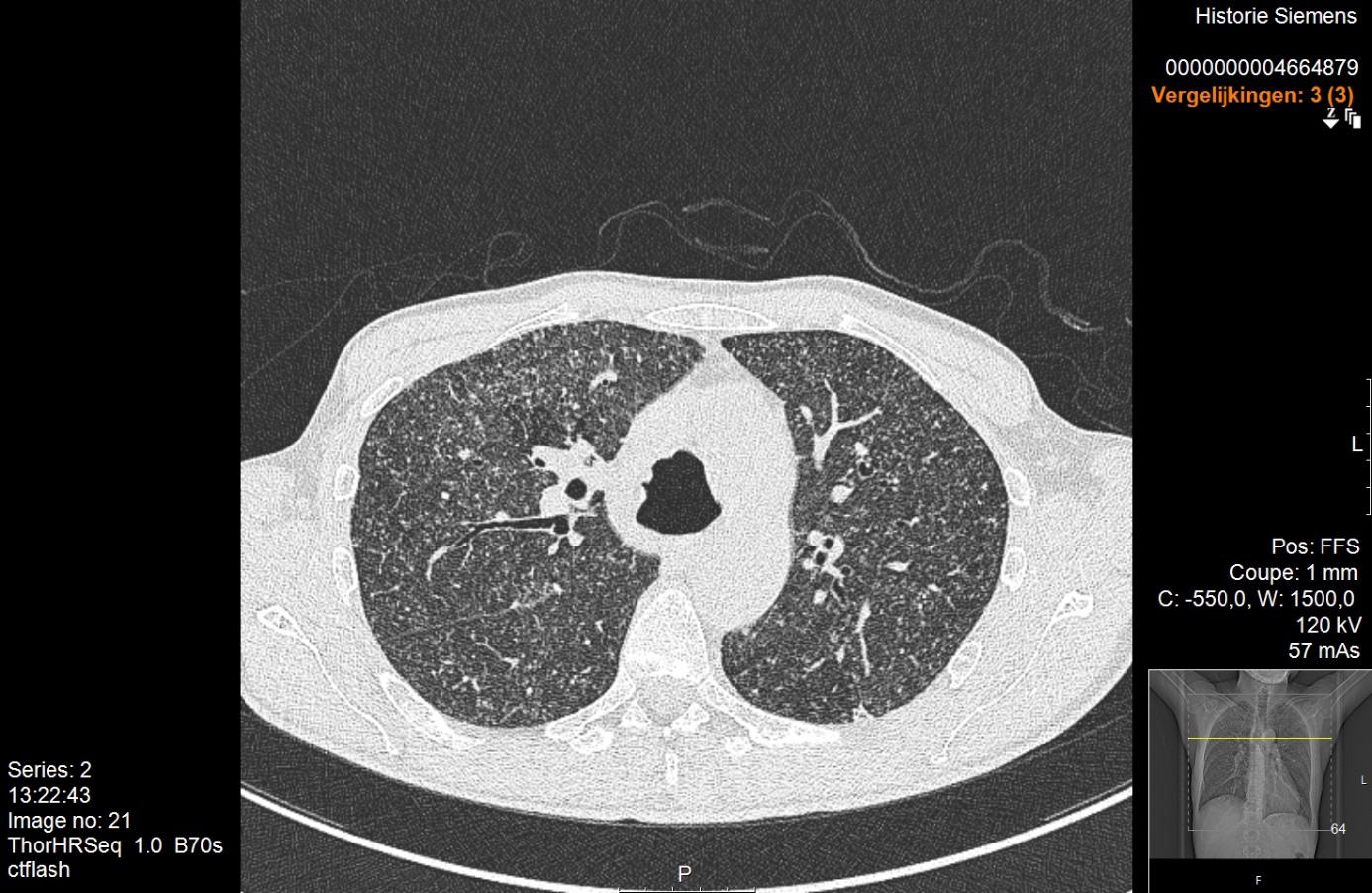
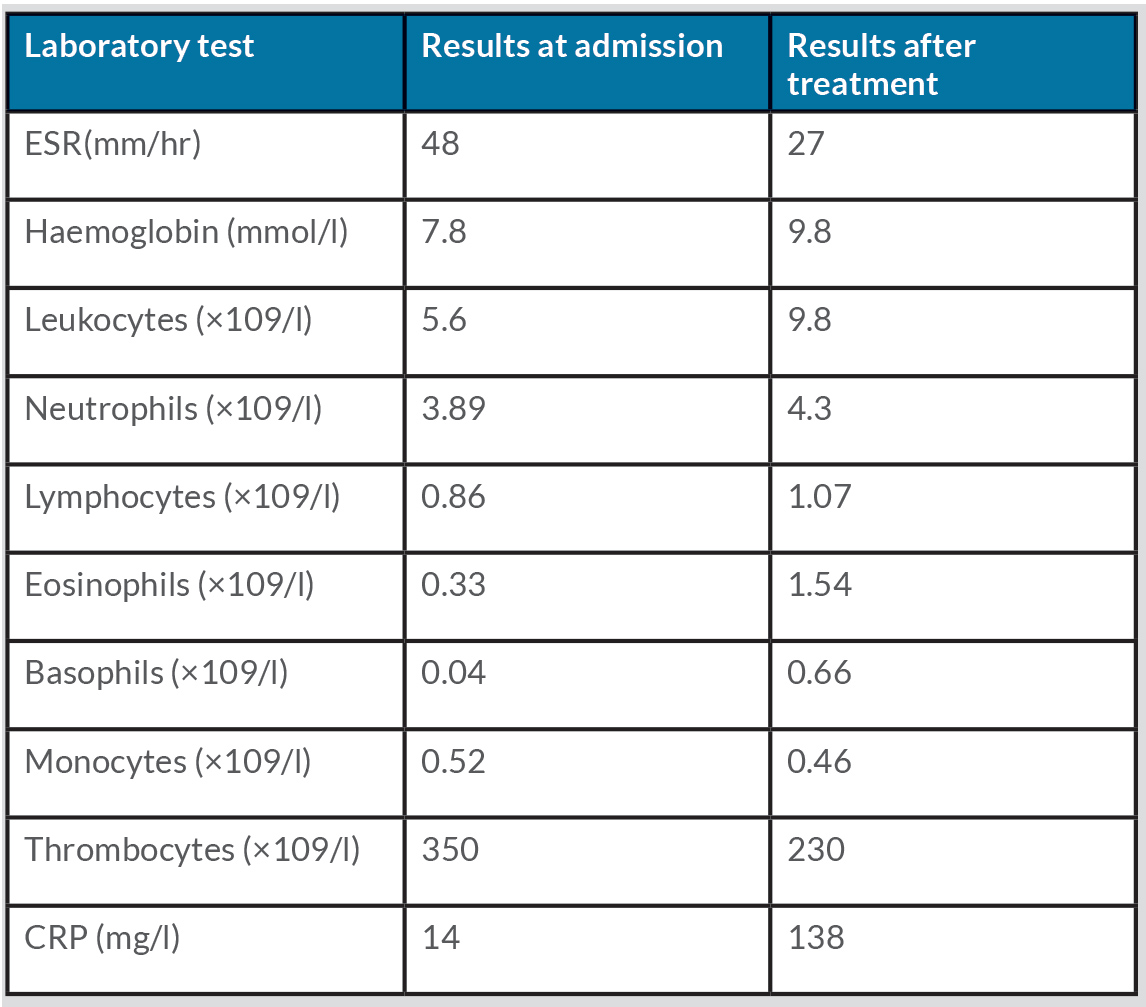
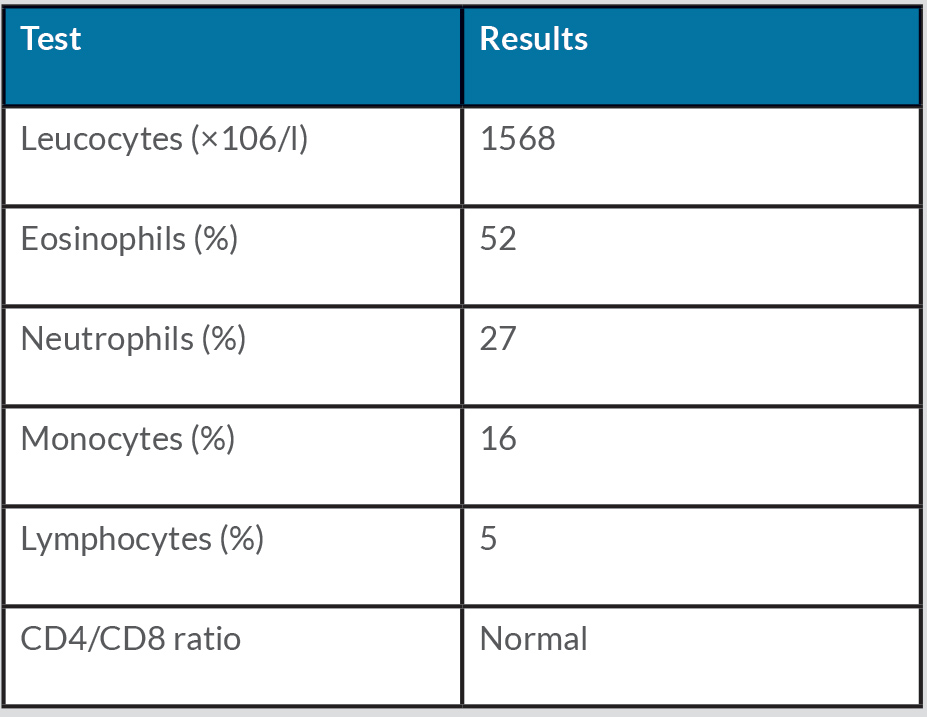
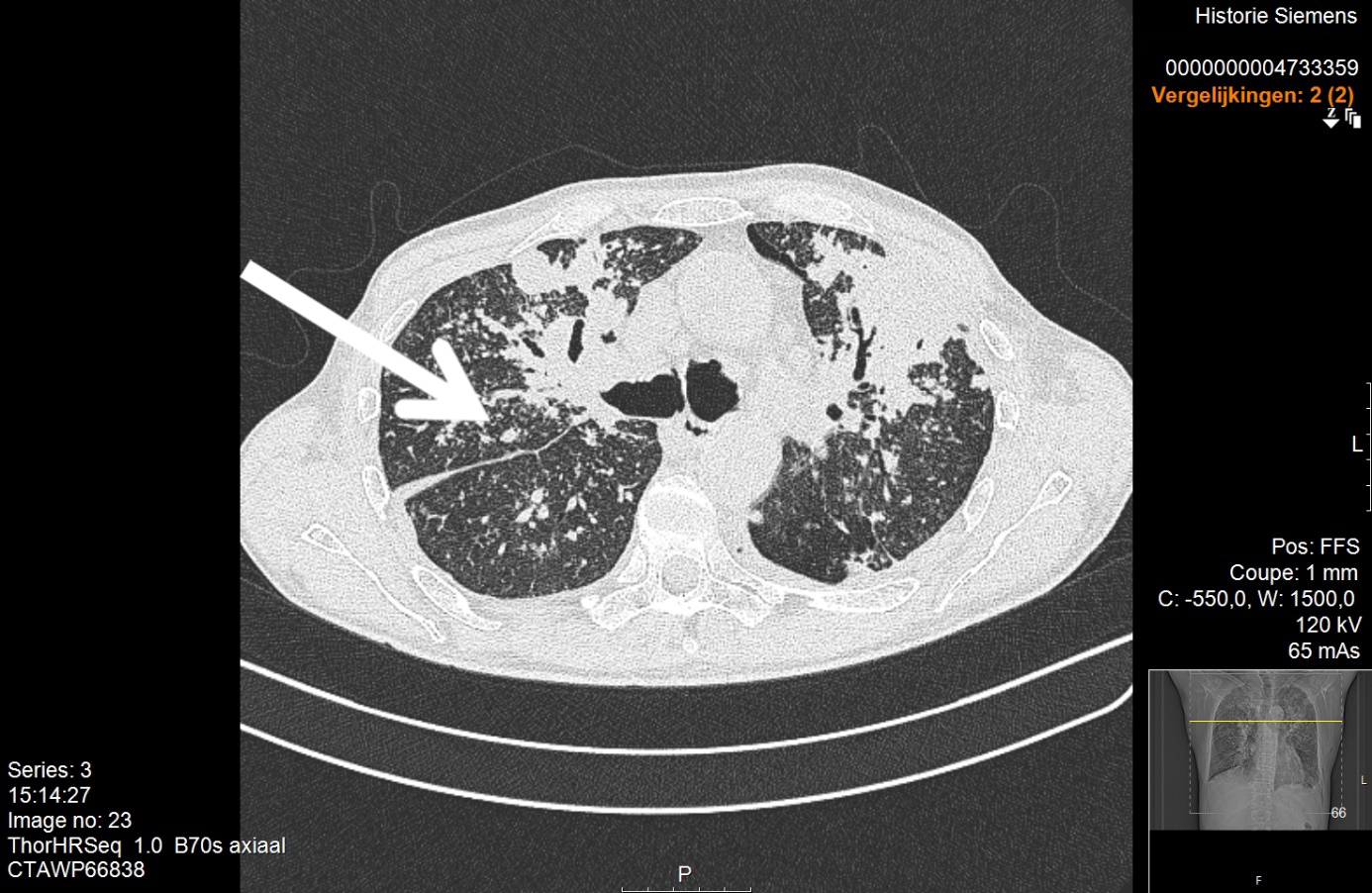
Physical examination was normal except for mild oxygen desaturation (91%). Laboratory results revealed increased inflammatory markers with eosinophilia (Table 1). High-resolution computed tomography (HRCT) revealed bilateral randomly distributed millimetric nodules (Fig. 3). Bronchoalveolar lavage fluid (BALF) analysis demonstrated a marked elevation of eosinophils (Table 2). The galactomannan index in BALF and serum was below the threshold for invasive pulmonary aspergillosis (IPA). Microbiological cultures for bacteria, mycobacteria and fungi in BALF were negative. Stool cultures for parasites were also repeatedly negative. Extended infectious serology and auto-immune serology were both negative including antineutrophil cytoplasmic antibodies. Total IgE levels rose to 617 kU/l.
Lung function tests excluded asthma, but revealed a restrictive deficit and a mild diffusion impairment.
Table 1 Laboratory results at admission and after treatment
Table 2. Bronchoalveolar fluid analysis
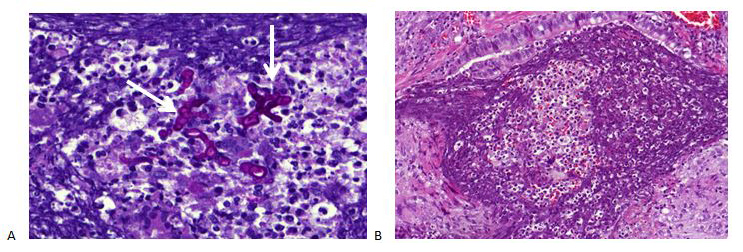
The galactomannan index in BALF and serum was below the threshold for invasive pulmonary aspergillosis (IPA). Microbiological cultures for bacteria, mycobacteria and fungi in BALF were negative. Stool cultures for parasites were also repeatedly negative. Extended infectious serology and auto-immune serology were both negative for antineutrophil cytoplasmic antibodies. Total IgE levels rose to 617 kU/l. Lung function tests excluded asthma, but revealed a restrictive deficit and a mild diffusion impairment. The patient was referred to a haematologist who excluded a hyper-eosinophilic syndrome. This patient had interstitial pneumonia with very high eosinophilia in BALF and peripheral blood. In the absence of an alternative diagnosis, the working diagnosis was chronic eosinophilic pneumonia, although the radiological pattern was extremely atypical. Treatment with corticosteroids was started. However, after 4 weeks of treatment, clinical deterioration was noted with the occurrence of night sweats and chills. HRCT showed progression with the formation of consolidations (Fig. 4). A lung biopsy was performed after multidisciplinary discussion. This demonstrated extensive inflammation with granulomas and the presence of fungal hyphae (Fig. 5). Aspergillus fumigatus was isolated. The patient was diagnosed with IPA and hospitalized for intravenous treatment with voriconazole.
Figure 4. HRCT after 4 weeks of treatment with prednisone showing diffuse bilateral consolidations
Figure 5. Histological analysis shows the presence of fungal hyphae (A, indicated with a white arrow) and a granuloma (B)
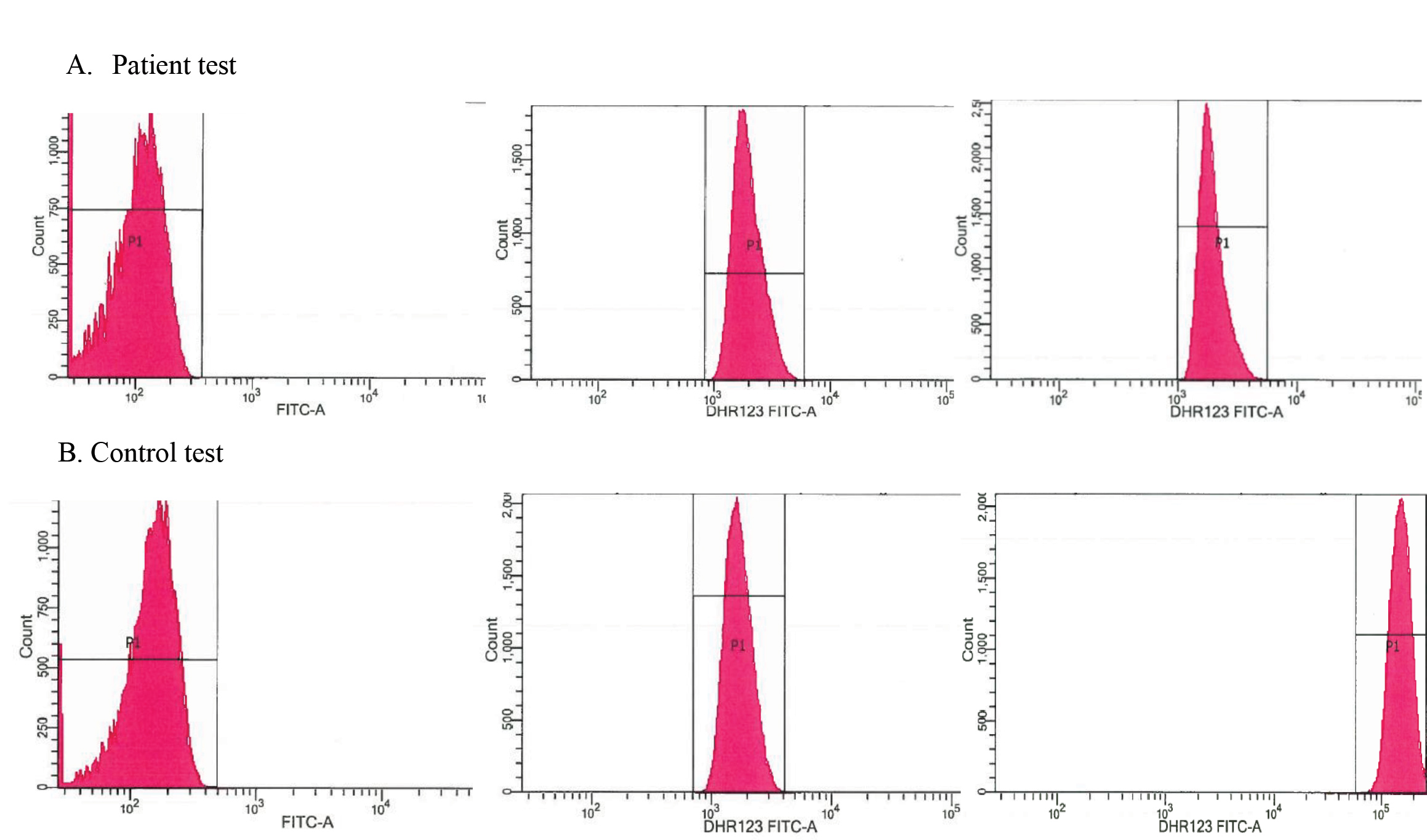
The presence of IPA was unexpected and extensive investigation for underlying immunodeficiency was initiated. All routine tests (blood cell count and differentiation, immunoglobulins, complement factors, mannose-binding lectin, HIV serology, abdominal ultrasound) were normal. An extensive literature search for rare causes of immune disorders suggested the possibility of CGD. A DHR 123 test in the haematological department was arranged and was positive for CGD (Fig. 6). Genetic analysis revealed a homozygote GT-deletion in the p47phox (NCF1) gene. The patient was diagnosed with the autosomal form of CGD complicated with Aspergillus infection. Voriconazole was continued, prophylactic antibiotic therapy was started and the patient was referred for genetic counselling. Stem cell transplantation was not an option at that time due to the Aspergillus infection. Under treatment, the patient’s symptoms, laboratory results (Table 1) and CT scans improved and he now attends 6-monthly follow-up visits in our clinic.
Figure 6. Flow cytometric dihydrorhodamine (DHR) 123 assay results in our patient (A) and a healthy control (B). Y-axis indicates neutrophil cell count, x-axis indicates fluorescence intensity of neutrophils. (A) Left: The MFI (mean fluorescence intensity) of non-stimulated neutrophils is 1.960. Middle: The MFI of unstimulated neutrophils probed with the DHR123 dye. Right: By stimulation of phorbol myristate acetate (PMA) neutrophils produce superoxide radicals resulting in the oxidation of DHR123 to fluorescent R123. The MFI of neutrophils stimulated by PMA is 1.945. This indicates deficient neutrophil oxidizing activity. (B) Left: The MFI of non-stimulated neutrophils is 1.677. Middle: The MFI of unstimulated neutrophils probed with the DHR123 dye. Right: The MFI of neutrophils stimulated by PMA is 150.275. This indicates normal oxidation and fluorescence of DHR 123 by the neutrophils.
DISCUSSION
We present this case to highlight the possibility of CGD in patients with Aspergillus infection, although it is very uncommon as the first presentation of this disease. Furthermore, we discuss the challenges of diagnosing IPA, especially in case of atypical or absent host factors.
Pulmonary aspergillosis is often diagnosed in patients with pre-existing lung disease or known immunodeficiency. This patient was presumed to be immunocompetent, although retrospectively, a history of recurrent otolaryngological infections could have raised suspicion.
We carried out an extensive search for immunodeficiency but did not consider CGD because of its rarity. However, CGD and other phagocytic disorders should be considered as the underlying cause of invasive aspergillosis, given the key role of the innate immunity in defence against Aspergillus species [2, 3]. The recommended DHR 123 test to diagnose CGD can be performed in routine settings within 1 day. Our hospital has now implemented this test and offers it to other hospitals in the region.
We were faced with a couple of diagnostic challenges because of the atypical presentation and misleading laboratory results, leading to a diagnostic delay. Because galactomannan was not detected in serum or in BALF, and there were no host factors, we excluded Aspergillus infection. We also were misled by the remarkably high pulmonary eosinophilia, which could not be explained by alternative diseases such as a parasite infection, eosinophilic granulomatosis with polyangiitis, or hypereosinophilic syndrome. This is not the first report of IPA in adulthood with marked eosinophilia as the first presentation [4]. It has been suggested that eosinophilic inflammation can be a response to Aspergillus infection. In animal models, eosinophils have been described as playing a key role in A. fumigatus lung infection and causing lung damage due to a cytokine storm [5]. Also, the deficient nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) system in CGD can lead to increased expression of eosinophils by a positive feedback mechanism. Case reports have been published which describe a hypereosinophilic inflammatory state in patients with CGD [6].
The negative predictive value of a BAL galactomannan finding to exclude Aspergillus infection in patients with low a priori risk, as in our case, is not validated. The sensitivity of a BAL galactomannan is estimated to be 90%, but those studies were all performed in patients with probable invasive aspergillosis and multiple host factors [7–9]. In immunocompetent patients, the sensitivity is probably lower [10].
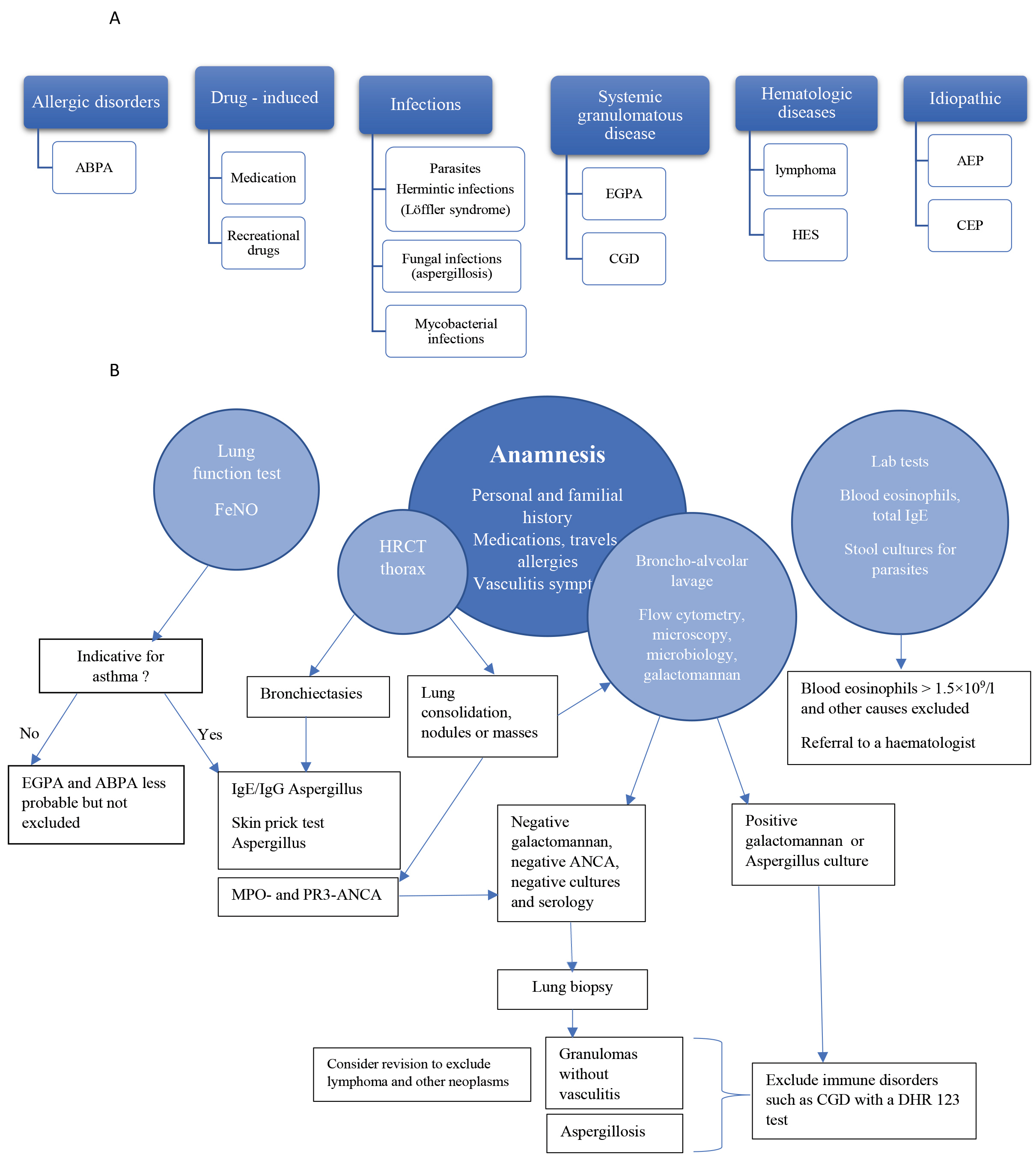
Health professionals should consider IPA in selected patients with unexplained pulmonary infiltrates and pulmonary eosinophilia even in case of a negative galactomannan test and presumed immunocompetence. Figure 7 provides a guide for the diagnostic work-up of eosinophilic lung disease. Diagnosis relies primarily on characteristic clinical/imaging features with the anamnesis having a central role. Laboratory tests and BALF are useful to determine the degree of eosinophilia and aid the diagnosis of infections or vasculitis. Generally, a lung biopsy is not necessary but is strongly recommended in case of negative or discrepant results [11].
Figure 7. Causes and diagnostic work-up of eosinophilic lung disease. (A) Causes of eosinophilic lung disease. (B) Flowchart for diagnostic work-up in patients with pulmonary infiltrates with eosinophilia. ABPA, allergic bronchopulmonary aspergillosis; AEP, acute eosinophilic pneumonia; ANCA, antineutrophil cytoplasmatic antibodies; CEP, chronic eosinophilic pneumonia; CGD, chronic granulomatous disease; EGPA, eosinophilic granulomatosis with polyangiitis; FeNO, fractional concentration of exhaled nitric oxide; HES, hypereosinophilic syndrome; HP, hypersensitivity pneumonitis; HRCT, high resolution computer tomography; MPO, myeloperoxidase; PR3, proteinase 3.
After a critical re-evaluation of this case, we conclude that a lung biopsy could not have been avoided but should have been performed before the trial treatment with prednisone. In that way, we could have pursued the diagnosis of IPA sooner, as well as the search for an underlying immune disorder.
CONCLUSION
IPA in adulthood with marked eosinophilia can be the first presentation of CGD. This underlying disorder should be considered in the absence of more common causes of immunodeficiency in previously presumed immunocompetent patients with IPA.
Pulmonary eosinophilia without an evident cause could be a manifestation of IPA with or without underlying CGD.