ABSTRACT
Prostate cancer is the second most frequent malignancy in men worldwide. Despite the improvement in survival achieved by increasingly early diagnosis and advances in treatment, it is still associated with high mortality. Because of its molecular heterogeneity, there is a need to identify genetic alterations in order to apply targeted therapies. Increasing evidence suggests that the PARP inhibitor olaparib could have a significant synthetic lethal effect in prostate cancer with homologous recombination defects, such as BRCA1/2 mutations. It is not yet known if, under these circumstances, platinum-based chemotherapy induces higher response rates in prostate cancer. We present the case of a patient with BRCA2-mutated metastatic castration-resistant prostate cancer whose treatment sequence included carboplatin and olaparib.
LEARNING POINTS
- Metastatic castration-resistant prostate cancer (mCRPC) remains a lethal disease despite significant progress in treatment.
- The BRCA2 mutation is associated with worse survival and so timely genetic screening is important.
- Studies are needed to identify the best therapeutic sequencing strategy for mCRPC harbouring homologous recombination repair defects, which includes PARP inhibitors and platinum.
KEYWORDS
Metastatic prostate cancer, BRCA2 germline mutation, PARP inhibitor, olaparib, carboplatin, BRCA2-mutated metastatic castration-resistant prostate cancer
INTRODUCTION
Prostate cancer is the second most common cancer in men and the fifth leading cause of death worldwide [1]. The incidence of prostate cancer rises in men over the age of 50. The next strongest risk factor is family history, identified in approximately 20% of patients [2].
Olaparib is a poly adenosine diphosphate ribose polymerase (PARP) inhibitor that induces synthetic lethality in tumour cells expressing defects in homologous recombination DNA repair (HRR), as seen in BRCA mutations. PARP inhibitors seem to induce clinical response in patients with metastatic prostate cancer with BRCA germline mutations similar to those observed in ovarian and breast cancer [3].
Platinum-based chemotherapy is one of the first research areas directed at patients with prostate cancer with HRR defects. A few cases have been described where olaparib and platinum were used for the treatment of metastatic castration-resistant prostate cancer (mCRPC) carrying a BRCA2 germline mutation.
CASE DESCRIPTION
A 45-year-old Caucasian man was diagnosed with prostate cancer. Although he had no relevant personal background, he had a strong family history of cancer. His mother had died at 38 years of age with breast cancer (diagnosed at 33). His father had lung cancer and his grandfather (on his mother’s side) had died in his 80s with lung cancer. He had an aunt (also on his mother’s side) who had died at the age of 57 from metastasis of an unidentified primary carcinoma, and he had a cousin (on his father’s side) who had been diagnosed with breast cancer at age 45.
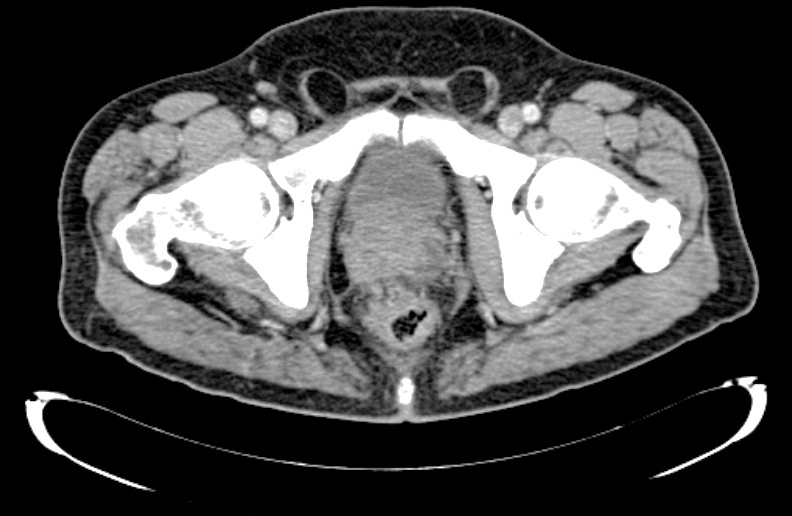
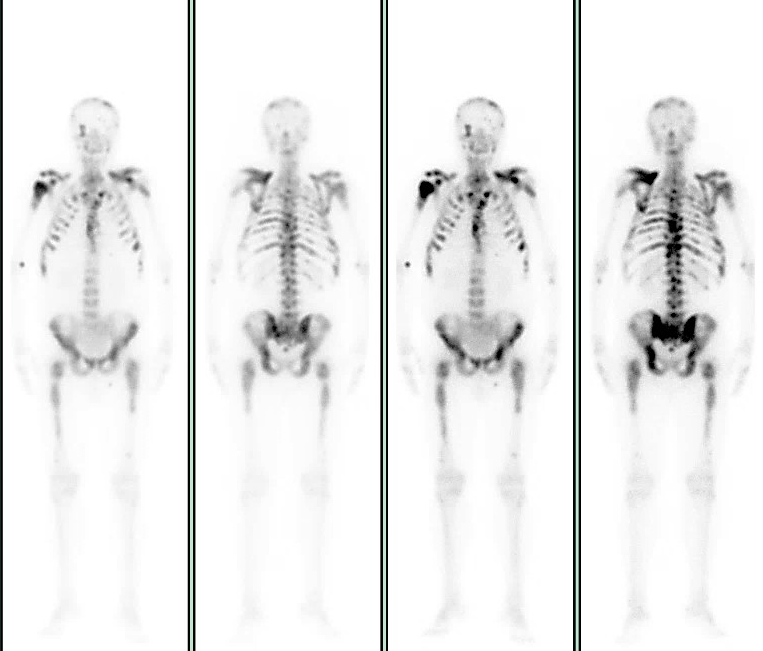
The patient had complained of cervical and low back pain for some months. A computed tomography (CT) scan was performed and revealed an enlarged prostate with irregular contours and heterogeneous density, compressing the bladder, and disseminated bone metastases, mainly in the pelvis, sacrum and dorsal and lumbar vertebral bodies and the right humerus, confirmed later by a bone scan (Figs. 1 and 2). The total prostate-specific antigen (tPSA) level was 352 ng/ml (reference value: 4 ng/ml) and a transrectal core prostate biopsy was performed. The results revealed prostate acinar adenocarcinoma Gleason 8 (4 + 4).
Figure 1. CT scan showing an enlarged prostate with irregular contours and heterogeneous density, which compresses the bladder
Figure 2. Bone scan confirming disseminated bone metastases, mainly in the pelvis, sacrum, and dorsal and lumbar vertebral bodies and the right humerus
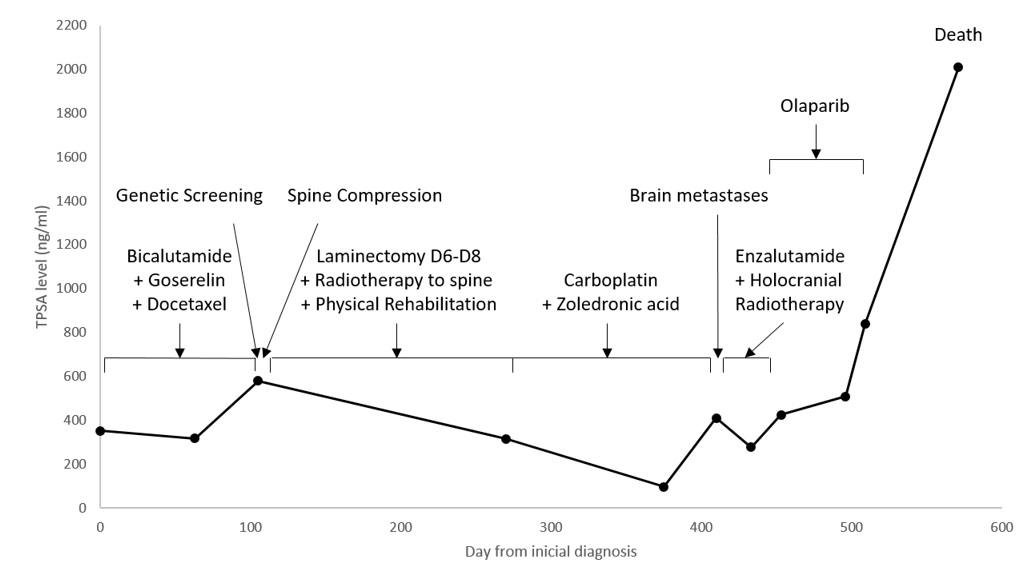
The clinical course of the patient is summarized in Fig. 3.
The patient promptly started injections of goserelin 10.8 mg every 12 weeks and bicalutamide 50 mg per day. The case was discussed in a multidisciplinary team meeting (MTM) and treatment with chemotherapy combined with hormone therapy, based on the data published in the STAMPEDE and CHAARTED clinical trials, was proposed [4, 5]. The patient started chemotherapy with intravenous docetaxel 75 mg/m2 every 3 weeks for six cycles, in combination with oral prednisolone 5 mg twice a day. The tPSA value decreased to 317 ng/ml during the third cycle but immediately began to rise. Suspension of chemotherapy was considered due to the appearance of hepatic metastasis during the fifth cycle. A tPSA value of 580 ng/ml was noted at that time.
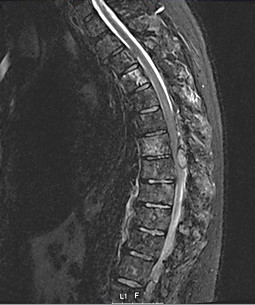
The patient was hospitalized the following month due to spinal compression. Spine magnetic resonance imaging (MRI) showed signs of diffuse secondary infiltration in all sacral, dorsal and lumbar vertebrae, the sacroiliac wings, and the ribs. From D6 to D8, a posterior predominantly epidural tissue component, with lateral extension, was seen compressing and causing deviation of the spinal cord (Fig. 4).
Figure 3. Clinical course of the patient. The serum tPSA level was measured for disease monitoring. The timeline and duration of different treatments are indicated, as well as the time point of genetic screening
Figure 4. Spine MRI revealing compression and deviation of the spinal cord caused by a posterior epidural tissue component at D6–D8
The patient was submitted to an emergent D6–D8 laminectomy.
Radiotherapy to the spine was performed on D5–D9 and L4–S2, with a total dose of 20 Gy delivered in five fractions with daily fractionation of 4 Gy.
The patient underwent a genetic screening test which revealed a BRCA2 mutation. Authorization for the use of olaparib was requested from the national medicine authority (Infarmed).
While awaiting authorization, the patient started monotherapy with intravenous carboplatin with a dose reduction (AUC 3) every 3 weeks, and intravenous zoledronic acid 4 mg every 3 months. tPSA decreased to 97 ng/ml but began increasing again shortly afterwards. After seven cycles of carboplatin, the patient showed signs of diplopia and a brain MRI was performed. The result revealed multifocal lesions affecting the calvaria and base with preferential involvement of the sphenoid body, invading the sella turcica, deforming the pituitary gland, and also infiltrating the middle cranial fossa and pressing on the temporal parenchyma.
Thus, it was decided in the MTM to interrupt carboplatin and start oral enzalutamide 160 mg per day due to lack of authorization of olaparib, and to perform holocranial radiotherapy. When the patient started enzalutamide, his tPSA was 409 ng/ml, which then decreased to 276 ng/ml but increased progressively thereafter. Holocranial radiotherapy was performed, delivering a total dose of 30 Gy in 10 fractions with daily fractionation of 3 Gy.
After 2 months, authorization was obtained and the patient started oral olaparib 400 mg twice a day. Due to anaemia grade 3 and thrombocytopenia grade 2 (according to CTC AE), it was necessary to reduce the dose of olaparib to 300 mg twice a day. However, after 2 months, olaparib had to be suspended due to worsening anaemia and disease progression, and it was decided to institute best supportive care.
The patient’s clinical condition worsened and 2 months later he was hospitalized and died shortly afterwards.
DISCUSSION
The DNA damage response is a vital pathway which ensures the survival of normal and malignant prostate cells as well as many important genes, such as BRCA1/2, ATM and PALB2. DNA damage response genes with mutations destabilize prostate cancer cells, which makes them more susceptible to cell death [6]. BRCA1/2 are tumour suppressor genes contributing to DNA damage repair through HRR, a process which repairs double-strand breaks. The nuclear PARP enzymes are involved in DNA repair, specifically single-strand breaks, through base excision repair pathways. In the absence of PARP enzymes, DNA defects can be repaired by other mechanisms. Even so, if a double-strand break cannot be repaired through homologous recombination, which occurs in BRCA mutations, the consequence is irreversible DNA damage and subsequent cell death. PARP inhibitors promote the progression of single-strand breaks to double-strand breaks, inducing synthetic lethality in cells with impaired homologous recombination mechanisms [3, 7].
It is estimated that the HRR defects occur in about 20–25% of patients with mCRPC, with mutations in BRCA2 and ATM being the most common [6, 8]. BRCA2 mutations increase the risk of prostate cancer for men under 65, and are also associated with more aggressive disease and worse survival [8]. Consequently, genetic counselling and screening is important to identify mutations in prostate cancer patients. Current European Society for Medical Oncology (ESMO) prostate cancer guidelines recommend genetic testing for BRCA2 and other genes involved in DNA damage response in patients with a family history of cancer, and should be considered in all patients with metastatic prostate cancer[9].
Olaparib was approved by the U.S. Food and Drug Administration for men with deleterious or suspected deleterious germline or somatic HRR gene-mutated mCRPC who have progressed following previous treatment with enzalutamide or abiraterone. The European Medicines Agency have limited its use to patients with mutations in BRCA1/2 genes.
We sought permission to use olaparib in our patient based on the results of a phase II study called TOPARP-A, that showed a high response rate, significantly longer radiological progression-free survival (rPFS) and increased overall survival (OS) in patients with DNA-repair gene defects [10]. More recently, PROfound was the first positive phase III biomarker-selected study evaluating a targeted treatment in patients with mCRPC, and reinforced the results of TOPARP-A [11]. This study assessed the efficacy and safety of olaparib versus enzalutamide or abiraterone in patients with mCRPC with alterations in any of 15 predefined genes with a direct or indirect role in HRR whose disease had progressed on previous therapy with a new hormonal agent. It reported various important conclusions, namely, a significant improvement in OS, rPFS and radiographic overall response rate in patients with mCRPC and HRR alterations with previous treatment undergoing olaparib compared with enzalutamide/abiraterone, despite substantial crossover from control therapy to olaparib [11].
While authorization was awaited, it was decided to begin treatment with carboplatin based on several studies which have shown that the status of the DNA damage repair pathway influences sensitivity to platinum-based chemotherapy. According to Cattrini et al., ‘Platinum generates DNA crosslinks that cannot be easily repaired when the HRR pathway is impaired, leading to cell death. This strategy has proven successful in treating breast and ovarian cancers with alterations in BRCA1/2.
Several case series and retrospective studies suggest that DNA damage response-deficient prostate cancer patients might benefit from this therapeutic approach, and many clinical trials are ongoing to assess the role of platinum-based chemotherapy in patients with such defects[12].
Further studies are needed to support the use of PARP inhibitors and platinum-based chemotherapy in mCRPC with defects in HRR pathway genes, with the agents administered in combination and/or in sequence. The best method of incorporation with existing therapies should be urgently examined [13].
Regarding our patient, the use of olaparib was, at that time, justified according to the TOPARP-A study. However, it is not known if he would have responded better if he had started earlier, when he would have been in a better clinical condition to continue the treatment for longer. In addition, as mentioned, the role of carboplatin is in this setting is not yet known, although it is believed that mCRPC patients with defects in the HRR pathway may respond well. Although some studies have shown a favourable response to PARP inhibitors and platinum-based chemotherapy in mCRPC, only a few specific clinical cases have been reported.
We await the conclusions of ongoing trials regarding the optimal combinations and sequence of treatments.