ABSTRACT
Extra-colonic Clostridioides difficile infection is rare. Here we describe a sickle cell disease patient with avascular necrosis who presented with persistent bacteraemia due to C. difficile and septic arthritis in a native knee joint, which responded very well to medical and surgical treatment but recurred multiple times within weeks of the cessation of antibiotics.
LEARNING POINTS
- Clostridioides difficile can rarely have a wide variety of extra-colonic manifestations.
- Patients with sickle cell disease may have a higher predisposition to extra-colonic C. difficile infection (CDI) with high mortality and recurrence rates.
- Intravenous metronidazole or vancomycin are the most widely used treatments for extra-colonic CDI.
KEYWORDS
Clostridioides difficile, anaerobic bacteraemia, septic arthritis, pyogenic arthritis, sickle cell disease
INTRODUCTION
Anaerobic bacteraemia is identified in less than 13% of all positive blood cultures [1]. Clostridioides difficile (formerly Clostridium difficile) is a spore-forming Gram-positive bacillus that is strictly anaerobic [2]. The usual presentation of toxigenic strains includes antibiotic-associated diarrhoea and pseudomembranous colitis [2]. Unusually, C. difficile can have extra-colonic manifestations that include appendicitis, spleen and liver abscesses, visceral abscess, scrotal abscess, cranial abscess, bone infection, prosthetic and periprosthetic joint infections, mycotic aneurysm, pericarditis and infective endocarditis, empyema and a spectrum of skin and soft tissue infections like cellulitis, vaginal abscess, perineal abscess and necrotizing fasciitis [3–6]. Here we describe a case of extra-colonic C. difficile infection (CDI) presenting as bacteraemia with septic arthritis in a sickle cell disease (SCD) patient.
CASE DESCRIPTION
A 21-year-old woman known to have SCD presented to the emergency room (ER) in January 2020 with a 4-day history of constant right knee pain and progressive swelling. She denied any history of fever, nausea or vomiting, diarrhoea or dysuria. Physical examination showed normal vital signs initially with a swollen, tender right knee all over the joint along with limitation of movement on passive and active motion. She also had weakness along her left side due to residual hemiparesis from a stroke 10 years previously. She had received a short course of ciprofloxacin 5 months before this presentation for a presumed urinary tract infection. However, she had not developed diarrhoea or abdominal pain since and had never been diagnosed with CDI or pseudomembranous colitis.
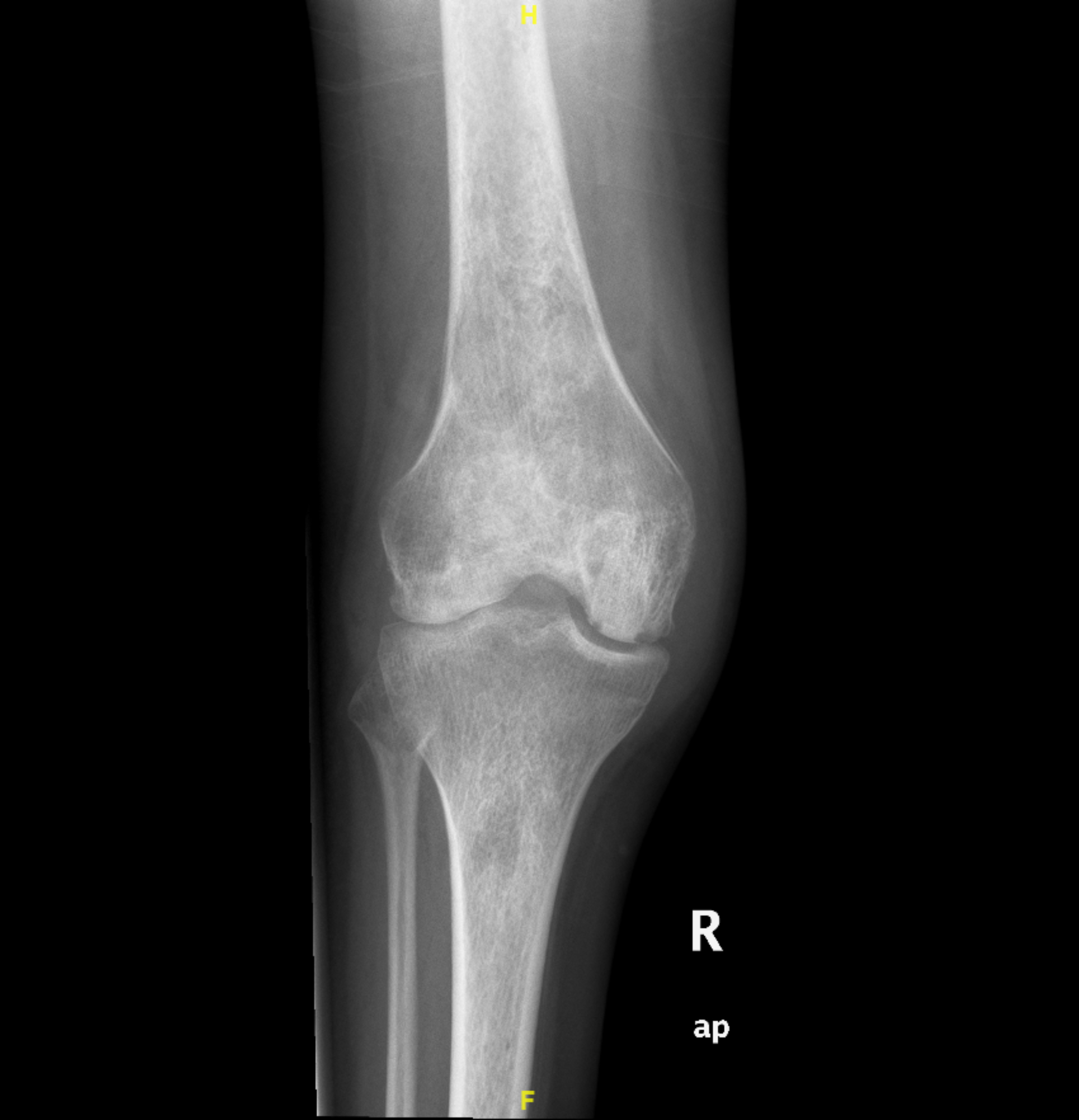
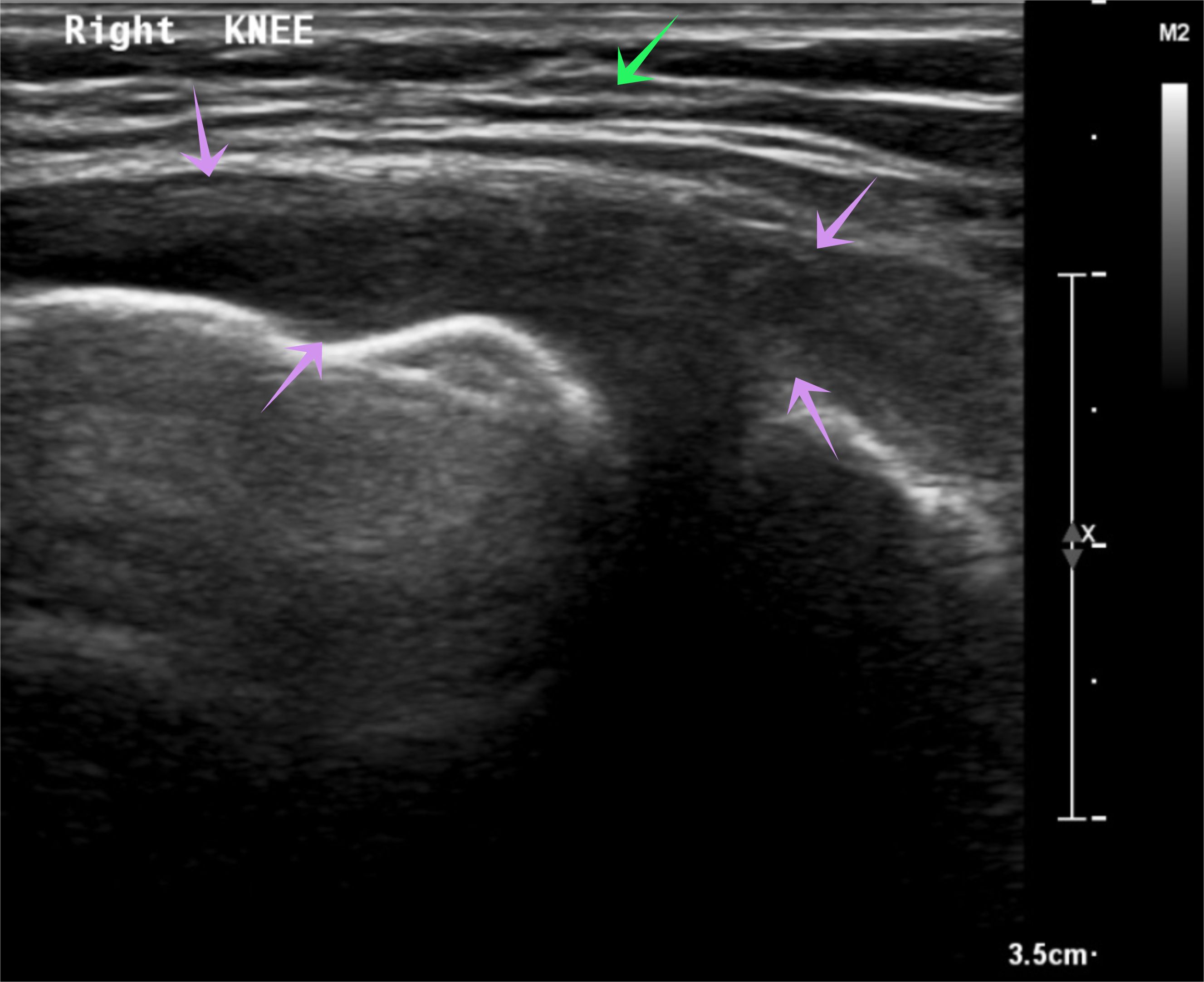
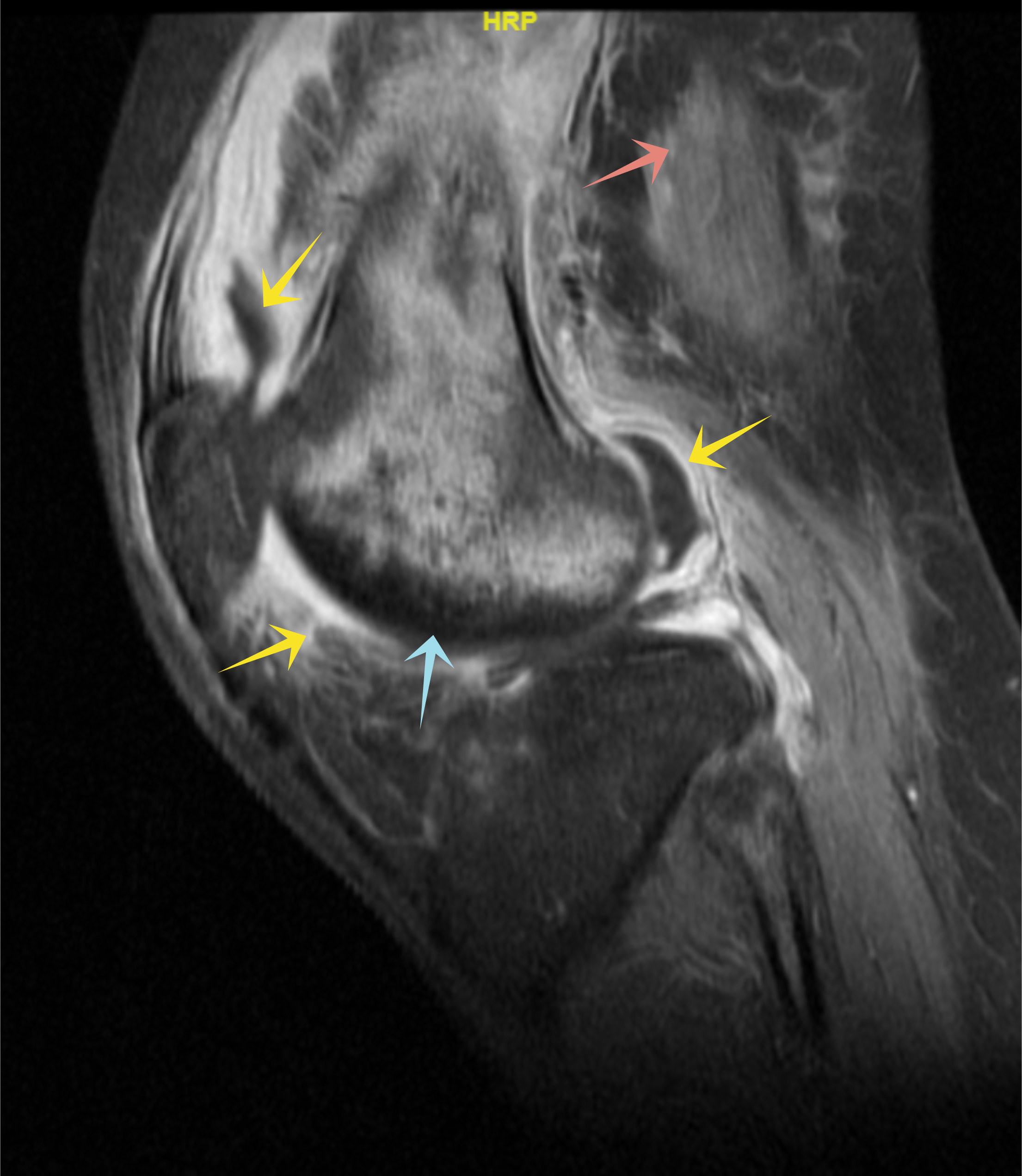
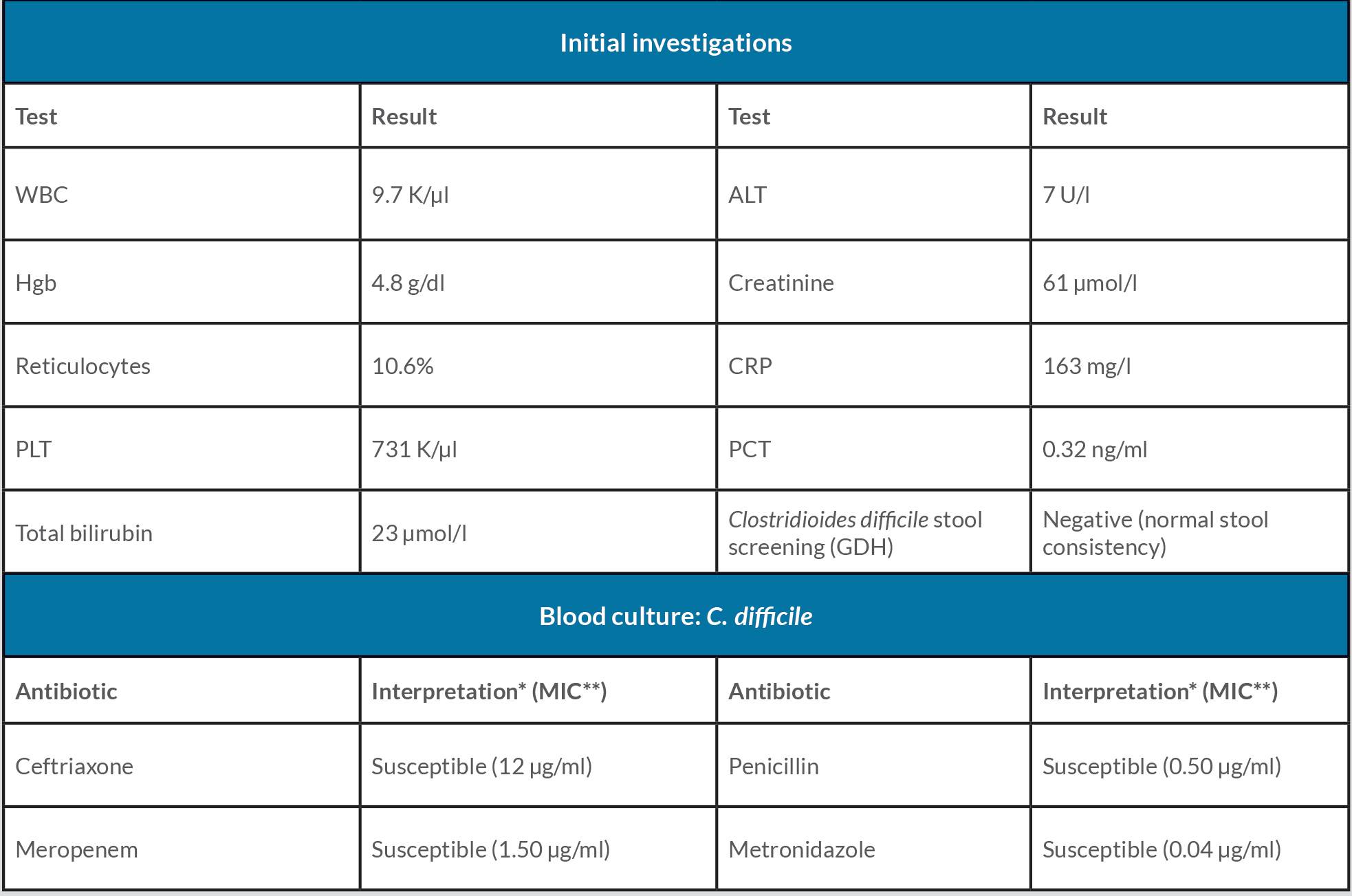
Initial investigation results are presented in Table 1. A right knee x-ray showed narrowing of the joint space (Fig. 1), while knee ultrasound showed a large effusion, synovial hypertrophy with debris and hyperaemia suggestive of septic arthritis (Fig. 2). Magnetic resonance imaging revealed a large amount of effusion on the right knee joint in addition to osteonecrosis and avascular necrosis (AVN) (Fig. 3).
Figure 1. Right knee x-ray. Anterior-posterior view of the right knee showing narrowing of the knee joint space
Figure 2. Right knee ultrasound. Right knee ultrasound showing large joint effusion (purple arrows) and hyperaemia with debris (green arrow) suggestive of septic arthritis
Figure 3. Right knee magnetic resonance imaging. Right knee MRI with evidence of large joint effusion (yellow arrows), subcutaneous oedema (red arrow), and evidence of osteonecrosis and avascular necrosis of the femur (blue arrow)
Table 1. Initial investigations and antibiotic susceptibilities
* As per the CLSI breakpoints.
** Calculated using the Epsilometer test (E-test; bioMérieux, Marcy l’Étoile, France)
ALT: alanine transaminase, CRP: C-reactive protein, GDH: glutamate dehydrogenase, Hgb: haemoglobin, PCT: procalcitonin, PLT: platelets, WBC: white blood cells.
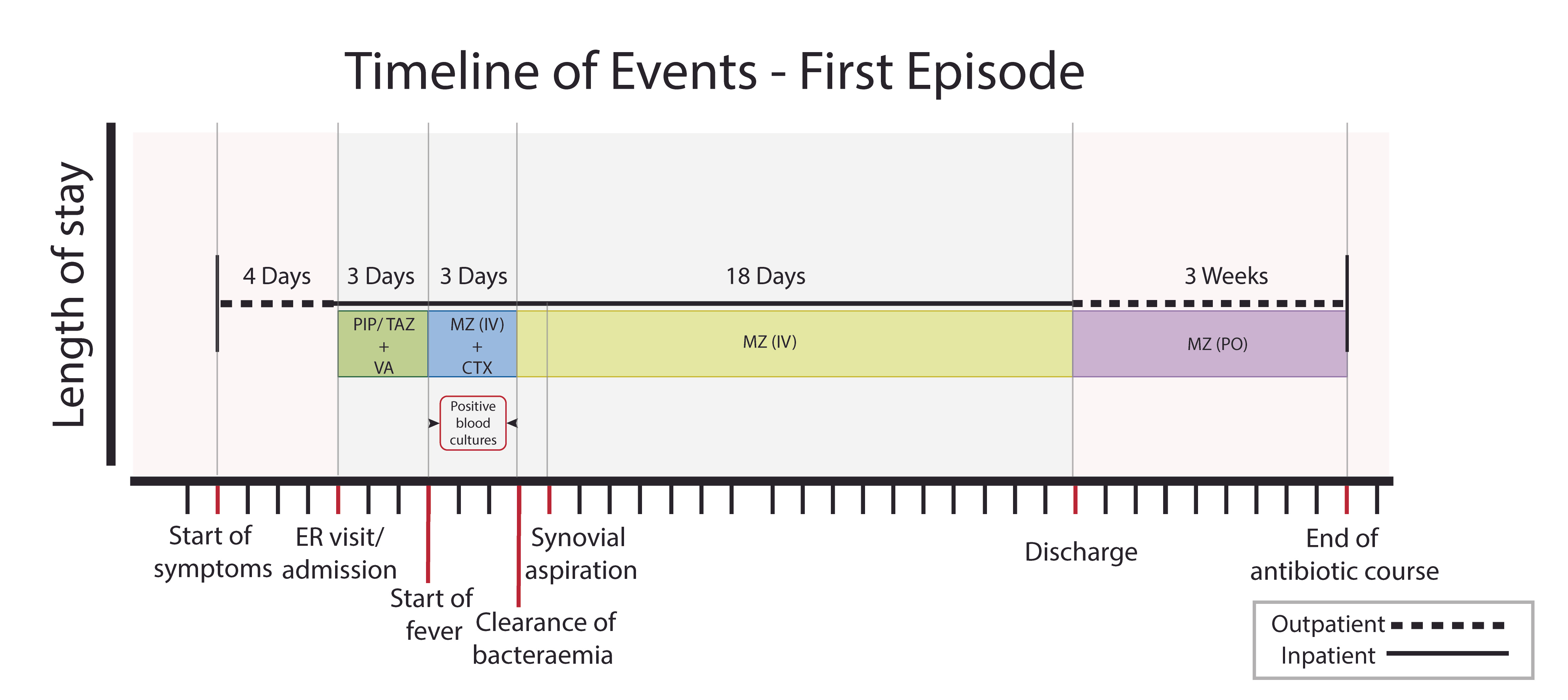
With a presumed diagnosis of septic arthritis and vaso-occlusive painful crisis, the patient was admitted and given intravenous fluids and pain killers. She was also started on empirical antimicrobials, including piperacillin/tazobactam and vancomycin (VA) intravenously (IV). Three days after admission, she developed a high-grade fever reaching up to 39°C that was associated with chills. Joint aspiration fluid was purulent and bloody (confirming the diagnosis of septic arthritis), but the amount was insufficient for a cell count and analysis. Synovial cultures (aerobic and anaerobic) were negative for bacteria even after enrichment (probably because it was done a few days after antimicrobials were started) (Fig. 4).
Figure 4. Timeline of events: first episode. Timeline of events showing the events of the first episode that started with symptoms at home followed by hospital admission through ER and empirical start of antibiotics, timing of the start of fever, the period of positive blood cultures, timing of blood culture clearance, timing of synovial aspiration, start of targeted therapy until discharge and the period at home after discharge until stopping antibiotics.
CTX: ceftriaxone, ER: emergency room, IV: intravenous, MZ: metronidazole, PIP/TAZ: piperacillin/tazobactam, PO: per os, VA: vancomycin
Gram staining was not done. Blood cultures grew a Gram-positive bacillus from anaerobic vials in less than 24 hours for 3 consecutive days (four vials), which was identified as C. difficile. This result was confirmed using MALDI-TOF (Bruker MALDI Biotyper, Bruker Daltonik, Germany). Antimicrobial susceptibility testing was done using the Epsilometer test (E-test, bioMérieux, Marcy l’Étoile, France) (Table 1). VA was replaced with metronidazole (MZ) and ceftriaxone IV. Bacteraemia resolved on the third day, so ceftriaxone was stopped and the patient continued on IV MZ alone for a total of 18 days from the first negative blood culture followed by 3 weeks of oral MZ.
Unfortunately, the patient’s symptoms recurred 6 weeks after discharge (2 weeks after she stopped her antimicrobial course); she was not compliant with the prescribed treatment. She was treated with another course of antimicrobials and three consecutive joint irrigations and debridement, but no organisms could be cultured from blood, purulent synovial fluid or intraoperative tissue from the knee. Intra-operative tissue samples were not sent for histopathological examination. The patient responded positively to the combined surgical and antimicrobial treatment and was discharged home in a stable condition. However, approximately 1 month after completing antimicrobial treatment, she experienced a third episode of possible septic arthritis. She visited our ER, where she, unfortunately, refused any further investigations or surgical interventions and left the hospital against medical advice and has been lost to follow-up since.
DISCUSSION
Antimicrobial use and advanced age combined with multiple comorbid conditions including liver cirrhosis, heart disease, pulmonary disease, chronic dialysis and immunocompromised status, are among the most common risk factors for CDI in general [2], while malignancy has been observed to be a possible risk factor for extra-colonic CDI specifically [7]. On the other hand, risk factors for septic arthritis, in general, include joint factors that act as a nidus for the infection to develop such as pre-existing joint disease, joint surgery or the presence of a prosthetic joint, while host factors include old age, skin infection and immunocompromised status [8]. Multiple cases of reactive and septic arthritis associated with CDI from prosthetic and native large joints have been reported in the literature [2, 8]. Interestingly, native joint septic arthritis due to CDI has been almost exclusively reported in SCD patient who also had confirmed C. difficile bacteraemia or evidence of possible bacteraemia with sterile cultures primarily due to prior use of antimicrobials [8].
Although specific ribotypes and the secretion of certain toxins have been associated with C. difficile-associated diarrhoea and C. difficile colitis, this association has not been found in extra-colonic CDI [2, 7]; in fact, non-toxigenic strains of C. difficile tend to be detected more frequently in extra-colonic CDIs [4, 8]. It has been noticed that extra-colonic CDI is mostly polymicrobial where C. difficile is thought to enhance other bacteria but can also be the only detected pathogen [4].
Unlike C. difficile colitis, there are no guidelines for the treatment or duration of treatment for extra-colonic CDI [7]. There are also significant gaps in the knowledge required for developing such guidelines, like pharmacokinetic/pharmacodynamic studies for anti-C. difficile antimicrobial parameters in different extra-colonic tissues and precise interpretation of breakpoints for antimicrobials by the CLSI and or EUCAST committees for the most frequently used antimicrobials in CDI [7]. The most frequently used antimicrobials for extra-colonic CDI in the literature include MZ and VA or both with variable response rates, high recurrence rates and mortality [7]. Surgical intervention has been necessary to treat some of the reported cases [7]. Faecal microbiota transplantation has been observed to improve arthritis due to psoriasis and probably other types of immune-mediated arthritis [9].
In our patient, the clinical presentation together with the risk factors strongly suggest septic arthritis type B according to the modified Newman’s criteria [10]. The link between C. difficile bacteraemia and septic arthritis in this patient could be confirmed if it were possible to perform advanced molecular sequencing from the purulent fluid aspirated from the joint, but unfortunately this technique is not yet available for clinical use outside of advanced research laboratories.
The association between SCD and extra-colonic CDI (septic arthritis with or without bacteraemia specifically) should be considered when treating similar patients, especially if they have been exposed to multiple antimicrobials which decrease the yield of the cultures leading to diagnostic dilemmas.