ABSTRACT
Platypnoea-orthodeoxia syndrome (POS) is a rare disorder and its pathophysiology has puzzled clinicians for years. Few cases of POS are described in COVID-19 patients in the literature, with a high variability of conditions related to the syndrome.
In this article, we report the case of a patient admitted to our hospital for SARS-CoV-2 interstitial pneumonia, who developed POS during the hospitalization.
LEARNING POINTS
- Platypnoea-orthodeoxia syndrome (POS) is a possible and rare condition associated with SARS-CoV-2 pneumonia.
- A prompt diagnosis of POS is important in order to start careful and adequate oxygen supplementation.
- Clinical data on COVID-19 evolution in patients with POS and possible therapeutic and rehabilitative strategies are not available in the literature.
KEYWORDS
Platypnoea-orthodeoxia syndrome, COVID-19, SARS-CoV-2
INTRODUCTION
Coronavirus disease 2019 (COVID-19) represents the worst pandemic in several decades, challenging healthcare systems through a sudden increase in hospitalizations for pneumonia with multi-organ disease. COVID-19 has a wide spectrum of clinical severity [1, 2].
Platypnoea-orthodeoxia syndrome (POS) is a rare disorder characterized by both dyspnoea and arterial desaturation that occurs when sitting or standing, with improvement in supine position [3]. The drop in oxygen saturation is considered significant when the partial pressure of oxygen (pO2) falls greater than 4 mmHg or arterial oxygen saturation (SaO2) falls more than 5% from supine to upright position. The pathophysiology of POS has puzzled clinicians for years; its real prevalence is still unknown and the precise mechanisms generating POS remain elusive in several patients [4, 5].
The most common cause is the mixture of deoxygenated venous blood and oxygenated arterial blood, through an interatrial right-to-left shunt such as a patent foramen ovale (PFO), an atrial septal defect or an atrial septal aneurysm [3, 6]. Extracardiac aetiologies include intrapulmonary arteriovenous malformations, lung parenchymal disease and cirrhosis leading to hepatopulmonary syndrome [4, 5].
Patients with POS develop dyspnoea when changing from a supine to a sitting/upright position, with a related drop in oxygen saturation; these gas exchange abnormalities and clinical manifestations resolve when the patient returns to a recumbent position. Careful and adequate oxygen supplementation alongside the removal of the underlying cause improve this condition.
Few cases of POS are described in COVID-19 patients, with a high variability of conditions related to the syndrome. We report a case of a patient admitted to our hospital for SARS-CoV-2 interstitial pneumonia, who developed POS during the hospitalization.
CASE DESCRIPTION
An 84-year-old woman presented to Spedali Civili’s Emergency Room for the onset of desaturation, associated with persistent weakness, fever and cough for 15 days. She was a non-smoker and gave no history of chronic lung disease.
Laboratory parameters including a haemogram and other samples are displayed in Table 1.
At admission leucopenia and mild anaemia were detected, as was an increase in C-reactive protein with normal liver and renal function. Her arterial blood gas analysis (ABG) performed in ambient air showed pH 7.48, pO2 77 mmHg without hypercapnia. The patient had a positive swab for SARS-CoV-2, and a chest x-ray showed a bilateral interstitial pneumonia. After admission, oxygen supply by nasal cannula was given because of desaturation.
The patient was treated with steroids, empirical antibiotic therapy (ceftriaxone and azithromycin), antithrombotic prophylaxis with enoxaparin (4,000 IU/day) and incremental oxygen therapy because of the worsening of respiratory failure. She underwent a repeat chest x-ray at Day 5 that showed a worsening of the interstitial pneumonia.
From Day 7 to Day 24 the patient needed non-invasive mechanical ventilation (NIMV) and values of positive pressure were modulated according to respiratory failure and barotrauma that occurred. The oxygen supply was gradually reduced, and the patient started a physiotherapy programme along with her medical condition improvement.
Within that context, a drop in oxygen saturation was observed in the sitting position; ABGs performed both supine and 10 minutes after sitting with the same oxygen supply confirmed gas exchange abnormalities. This evidence was measured at 2 different moments of hospitalization on Day 24 and on Day 39 (Table 1).
The chest computed tomography (CT) scan with contrast agent showed bilateral ground-glass attenuation especially in the lower lobes, in the absence of pulmonary embolism (Fig. 1).
Figure 1. Chest CT scan. On the left side are shown chest CT scans from lungs’ apex to base in the lung window, while on the right side are shown scans of the same levels in the soft-tissue window
Table 1. The patient’s clinical and laboratory characteristics over the hospitalization
pO2: Peripheral oxygen saturation; WBC: white blood cells; CRP: C-reactive protein; LDH: lactate dehydrogenase; ABG: arterial blood gas analysis;
pCO2: partial pressure of carbon dioxide; pO2: partial pressure of oxygen; SaO2: arterial oxygen saturation; FiO2: fraction of inspired oxygen; NC: nasal cannula;
VM: Venturi mask; HFNC: high-flow nasal cannula; C-PAP: continuous positive airway pressure; NIMV: non-invasive mechanical ventilation
We performed bubble contrast echocardiography which revealed no intracardiac shunts: in this way we could exclude cardiological causes of the patient’s POS. The patient continued to be treated with decremental steroid therapy and multimodal oxygen therapy: oxygen quantity was higher when sitting and during physiotherapy.
She tested negative for SARS-CoV-2 for 2 swabs obtained on Day 38 of hospitalization.
POS progressively improved with physiotherapy; the patient was discharged on room air both in supine and sitting position after 46 days of hospitalization and was referred to a facility to continue the rehabilitative course.
DISCUSSION
This article describes the case of a patient affected by SARS-CoV-2 pneumonia who developed symptoms configuring POS.
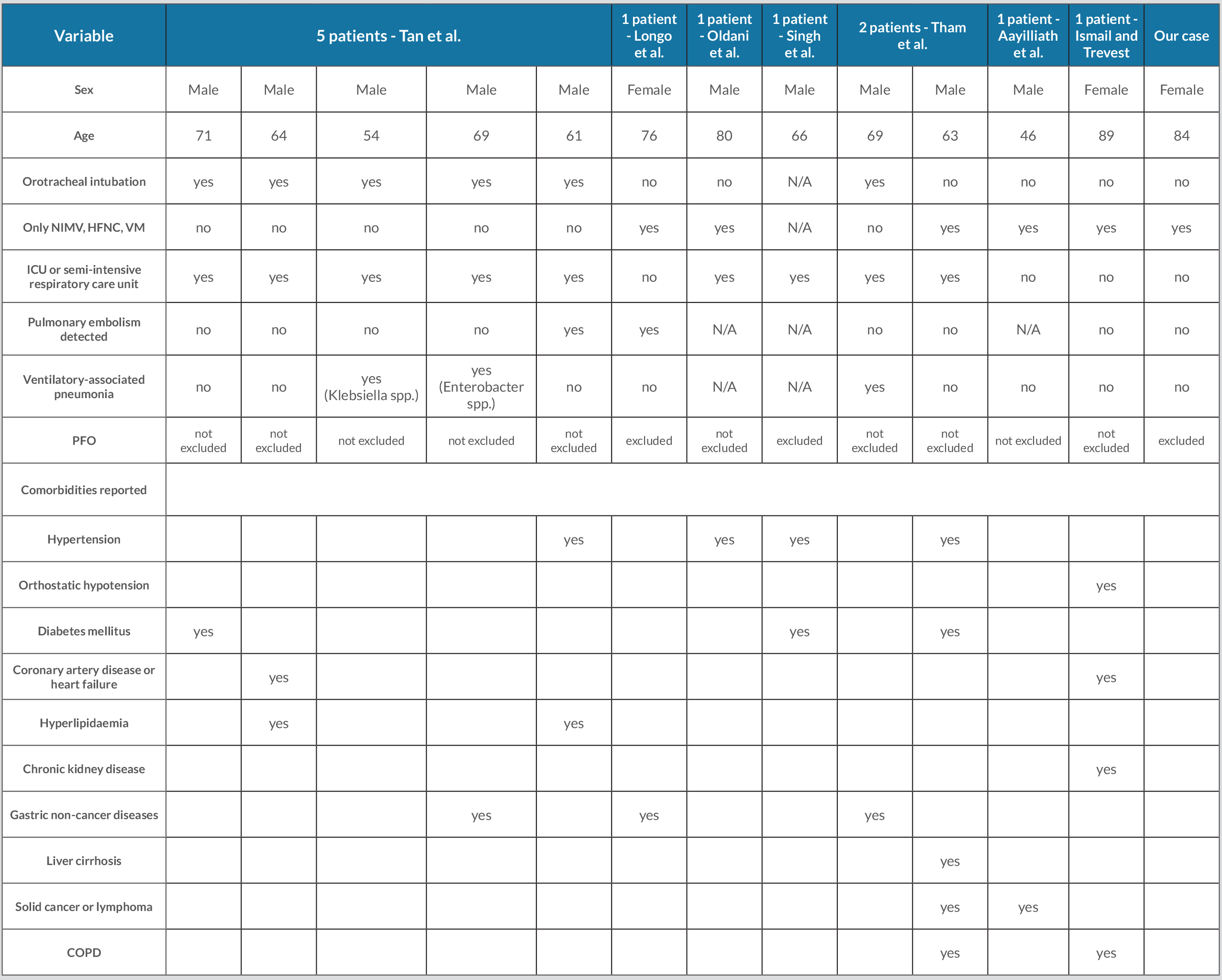
To the best of our knowledge, after a systematic and thorough literature search the coexistence of POS and COVID-19 was previously described by few authors [4, 7–12], showing some differences with our case (Table 2). We observe that 83% of the cases described in the literature were male and the median age was 67.5 years old, while our patient was an 84-year-old woman.
Table 2. Patient features for prior publications of POS in COVID-19
N/A: Not available; NIMV: non-invasive mechanical ventilation; HFNC: high-flow nasal cannula; VM: Venturi mask; ICU: intensive care unit; PFO: patent foramen ovale;
COPD: chronic obstructive pulmonary disease.
Of the cases known in the literature, 75% required hospitalization in intensive or semi-intensive care units and 50% of all cases needed intubation to support ventilation; conversely, our patient was treated only with NIMV. Therefore, it is unlikely that the exposure to orotracheal intubation could be ascribed as a precipitant factor for POS.
The differential diagnosis process includes the assessment of intracardiac shunting of blood and pulmonary embolism: bubble contrast echocardiography and a CT scan with contrast agent were performed and excluded heart and pulmonary arteries as causal factors. Pulmonary embolism was found in 17% of the cases described in the literature, contributing indeed only partially to POS in COVID-19. Cirrhosis and hepatopulmonary syndrome were not present in our patient, as in most patients previously described, ruling out a shared pathogenesis. Our patient had none of the chronic diseases described in other patients and any prior diseases associated with POS [3, 4].
Finally, 25% of the literature cases showed a contextual ventilator-associated pneumonia; however, in most patients and in our case, no other infectious diseases were detected except for SARS-CoV-2. The exact patho-physiological process of POS in COVID-19 remains a conundrum, given the wide variability of patients presenting this syndrome, according to published data. We suppose potential mechanisms similar to other parenchymal lung diseases with preferential involvement of lung bases [5]. Lower lung-predominant disease, confirmed on the CT scan in our patient, could have led to ventilation-perfusion (V/Q) mismatch, exacerbated by the orthostatic position: the redistribution of perfusion is not counterbalanced by consensual modification of alveolar ventilation, with a consequent increase in shunt percentage, gas exchange abnormalities and peripheral desaturation in the sitting position. In the upright position gravity increases blood flow in the lung bases more than in the apical regions and right ventricular preload is reduced, so alveolar pressure exceeds pulmonary arterial pressure at the lung apex.
Pneumonia base involvement and the resulting V/Q mismatch in the upright position could play a key role in the development of POS in COVID-19 [5]. That was demonstrated by Longo et al. [4] by performing a V/Q study through a SPECT/CT scan both in supine and sitting positions in a patient affected by SARS-CoV-2 pneumonia. When switching from supine to sitting position, a reduction both of the basal ventilation scan and the perfusion scan was observed, but the percent change for the ventilation scan was greater than that for perfusion.
Potential support for this hypothesis is provided by the reversible feature of POS according to COVID-19 improvement, enforcing its link with the acute phase of the disease and the hyperinflammation environment [13].
However, there are neither clinical data on COVID-19 evolution in patients with POS nor on possible rehabilitative strategies in the setting following acute respiratory failure, requiring non-invasive or invasive treatment. Prone position is an effective strategy to improve gas exchange in acute respiratory distress syndrome (ARDS) [14]. No predictor role of POS development was addressed to the pronation approach in COVID-19 [7], while Aayilliath et al. reported good tolerance of this strategy [11].
Further studies are needed to better understand the underlying mechanisms, in order to design appropriate therapeutic and rehabilitative programmes improving short- and long-term patient outcome.