ABSTRACT
SARS-CoV-2 causes severe acute respiratory distress and other clinical complications such as thromboembolic events and gastrointestinal tract disorders, which generally present with abdominal pain. In the case report, we describe a patient who had severe viral necrotizing pancreatitis associated with COVID-19 infection.
LEARNING POINTS
- In patients with COVID-19, gastrointestinal symptoms should raise suspicion of pancreatitis and additional evaluation should be carried out.
- Further research to investigate if COVID-19 is associated with acute pancreatitis is warranted.
KEYWORDS
COVID-19, pancreatitis, lipase, amylase, pandemic
INTRODUCTION
The COVID-19 pandemic began in March 2020, and has since spread worldwide. It is associated with respiratory distress and a wide range of symptoms such as fever, cough and shortness of breath. It is also associated with gastrointestinal (GI) symptoms including abdominal pain.
CASE DESCRIPTION
A 79-year-old woman, who is a known case of hypertension on oral cilnidipine and oral perindopril and seasonal bronchitis managed with inhalers, complained of fever and a sore throat. She did not consume alcohol regularly. A reverse transcriptase polymerase chain reaction (RT-PCR) test conducted 3 days later was positive for SARS-CoV-2. The patient also complained of abdominal pain and had a few episodes of vomiting. She presented to the hospital with confusion, restlessness and refusal of oral feeds.
She was admitted to the COVID isolation ward. Her symptoms worsened on day 5 and increased shortness of breath was noted on examination with bilaterally equal air entry and a few basal crepitations on auscultation. She had tenderness in the epigastric region of the abdomen. She was transferred to the ICU on day 6 because of increasing shortness of breath and deranged laboratory test results (Table 1). We initiated non-invasive ventilator (NIV) therapy to aid respiratory function and intravenous fluids were started to manage dehydration.
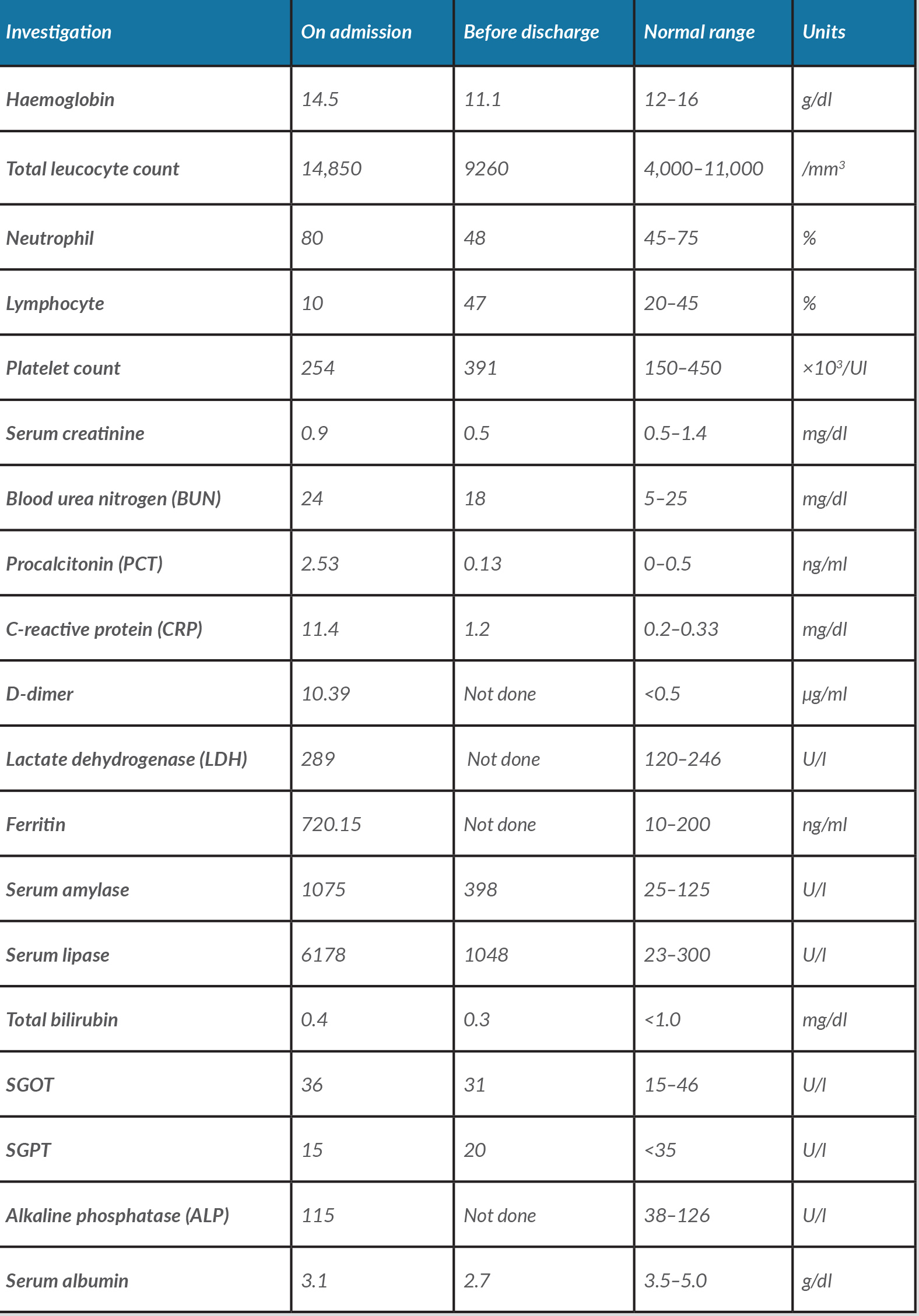
Table 1. Blood test results on admission and before discharge
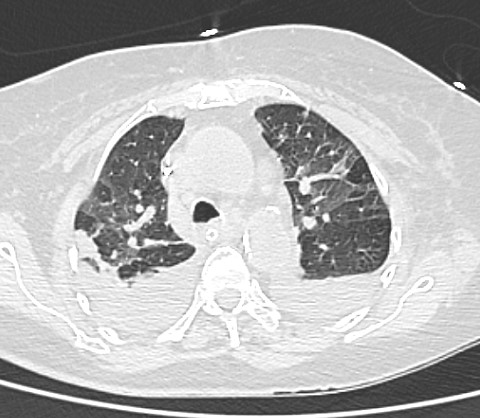
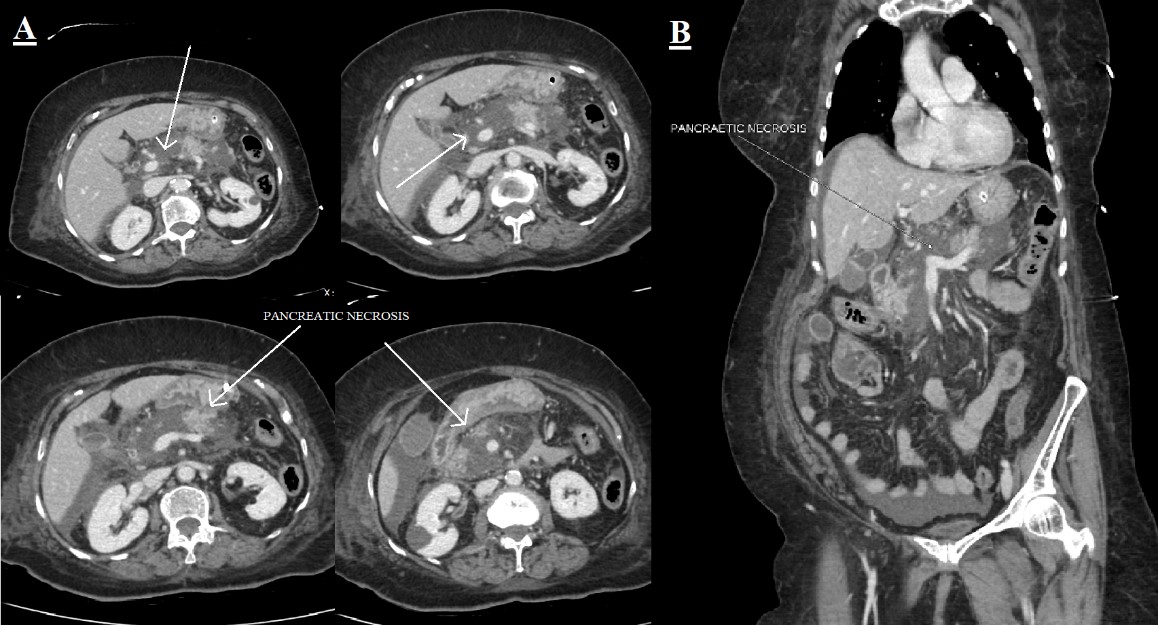
/div>A computed tomography scan of her chest, abdomen and pelvis showed ground-glass opacities in the bilateral upper lobes with a CO-RADS score of 6 and CT severity score of 4/25 (Fig. 1). A bulky pancreas with heterogeneous enhancement with non-enhancing areas suggesting necrotizing pancreatitis with partial filling defect in the distal splenic vein and at the confluence of the superior mesenteric vein was noted (Fig. 2).
Figure 1. HRCT scan of the chest showing bilateral ground-glass opacities and bilateral pleural effusion with collapse/consolidation. The CT severity score was 4/25
Figure 2. A CT scan of the abdomen and pelvis: (A) four axial plane views and (B) a coronal view showing a bulky pancreas with heterogeneous enhancement with non-enhancing areas suggesting necrotizing pancreatitis
We began treatment with intravenous antiviral therapy with remdesivir in accordance with our local hospital guidelines, as well as plasma therapy. The patient was also supported with parenteral nutrition and conservative management for the pancreatitis. She had an episode of upper GI bleeding so subcutaneous low molecular weight heparin was withheld, which increased the risk of venous thromboembolism. She had uncontrolled diabetes mellitus secondary to the inflamed pancreas. Her blood sugar level was managed with subcutaneous rapid acting insulin to maintain it below 200 mg/dl. She was intubated and ventilated under sedation on day 7 after respiratory symptoms worsened.
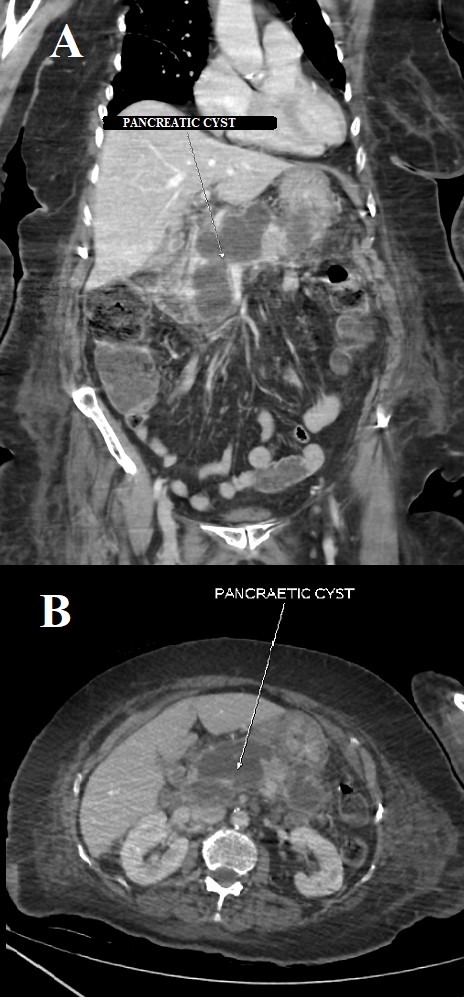
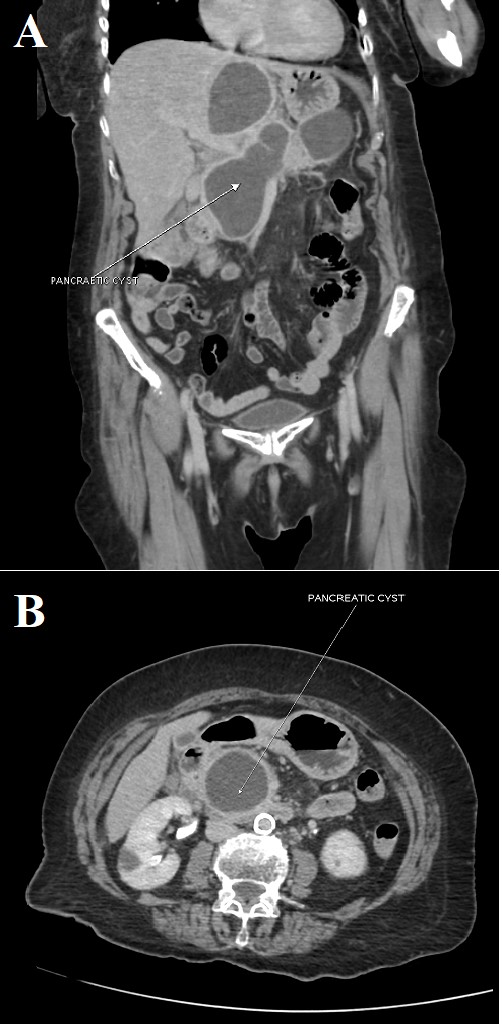
Her symptoms gradually improved and she was slowly weaned off sedation and the ventilator, and was extubated after 10 days of continuous ventilatory support. She required intermittent NIV support but her requirement for oxygen supplementation slowly decreased. She continued to complain of intermittent pain and tenderness in the abdomen. She was managed conservatively with intravenous fluids and a liquid diet. A repeat CT scan of her abdomen revealed a multiloculated collection with peripheral enhancement measuring 7.0×3.4×9.3 cm (SI×AP×transverse) with mass effect that might have caused the filling defect noted on the earlier CT scan (Fig. 3). She was finally discharged after her symptoms improved and was counselled about the need for future surgical intervention due to persistently elevated pancreatic enzymes and the presence of the cystic mass-like lesion. She was also advised to follow a low-fat and diabetic diet along with plenty of fluids at home.
After discharge, the patient still complained of intermittent abdominal pain, a few episodes of vomiting and loose stools. An outpatient-based CT scan of the abdomen and pelvis was repeated 4 months later and revealed a bilobed cystic mass lesion measuring 8.3×6.0 cm with septa, with internal echoes seen adjacent to the head and body of the pancreas; a similar lesion was also noted on the tail of the pancreas measuring about 5.5×4.5 cm (Fig. 4). The patient was readmitted for elective surgery. A cysto-jejunostomy with triple anastomosis was performed. The patient's symptoms resolved after the surgical procedure. Currently she has no complaints of abdominal pain, vomiting or loose stools.
Figure 3. A repeat CT scan of the abdomen and pelvis: (A) coronal view and (B) axial cut that shows a cystic mass formation/a multiloculated collection with peripheral enhancement measuring 7.0×3.4×9.3 cm (SI×AP×transverse)
Figure 4. A CT scan of the abdomen and pelvis 4 months later: (A) coronal view and (B) an axial cut showing a bilobed cystic mass lesion measuring 8.3×6.0 cm with septa, with internal echoes seen adjacent to the head and body of the pancreas; a similar lesion was also noted on the tail of the pancreas measuring about 5.5×4.5 cm
DISCUSSION
Many studies have reported acute pancreatitis in patients with co-existing viral infection such as mumps, cytomegalovirus, coxsackievirus B and influenza [1, 2], but the reasons for the inflammation are still unclear. A diagnosis of acute pancreatitis needs to fulfil at least two of the following three criteria: (a) abdominal pain, (b) elevation of serum amylase or lipase greater than three times the normal upper limit, and (c) diagnostic radiological imaging that suggests findings associated with pancreatitis [2, 3].
Previous studies have shown that COVID-19 may be associated with GI symptoms and have identified viral RNA in the GI tract. Several factors may be involved in inflammation of the pancreas such as pancreatic autodigestion, complement system activation of excessive leucocytes, disturbance in circulation, as well as acinar cell apoptosis and necrosis. Pancreatitis may develop as a result of inflammation and oedema that cause direct destruction of the acinar cells. This could lead to leaking of intracellular enzymes or precipitate apoptosis [2]. The angiotensin converting enzyme 2 receptor (ACE2) is also present in the GI epithelium which also provides an entry receptor for SARS-CoV-2[4].
A few case reports of acute pancreatitis in patients with COVID-19 have been published. One by Schepis et al. describes the presence of SARS-CoV-2 in a pancreatic pseudocyst from a patient with acute pancreatitis and COVID-19 [5, 6]. This virus has also been isolated from pancreatic tissue during autopsies [6, 7]. A quick search on PubMed with the keywords ‘pancreatitis’ and ‘COVID’ revealed several case reports. A report by Aloysius et al. presented the case of a 36-year-old woman with symptoms typical for COVID-19 as well as GI symptoms such as epigastric pain, who was diagnosed with acute pancreatitis and acute respiratory distress syndrome (ARDS) [1]. Another case report by Anand et al. presented a 59-year-old female patient diagnosed with COVID-19 and acute pancreatitis. This patient consumed very little alcohol and had a history of cholecystectomy. Her acute pancreatitis was reported to be idiopathic due to the lack of precipitating risk factors [2]. In addition, another report by Hadi et al. described three family members of whom two were diagnosed with acute pancreatitis following infection with SARS-CoV-2. Other causes of acute pancreatitis such as a history of alcohol consumption, biliary obstruction/gallstones, mineral level imbalance, drug use and trauma were ruled out [3]. Many other such case reports have discussed a possible link between pancreatitis and COVID-19.
However, in contrast, a few studies have demonstrated no significant increase in the incidence of acute pancreatitis during the current pandemic. A study published by Inamdar et al. analysed patients from 12 hospitals across the USA and reported a prevalence of 0.27% of acute pancreatitis in patients who also had COVID-19 [6, 8]. Another retrospective study by Miro et al. reported a frequency of 0.07% of acute pancreatitis across 50 Spanish emergency rooms [6, 9]. These findings indicate that it is unclear if there has been an increase in acute pancreatitis during this pandemic. According to the above studies, it seems unlikely that acute pancreatitis occurs frequently in patients with COVID-19.
CONCLUSION
No apparent cause for acute necrotizing pancreatitis was identified in our patient, although it may have had a viral aetiology due to ongoing infection with SARS-CoV-2. The presented case shows that it is important to measure serum levels of amylase and lipase in patients with COVID-19, especially if GI symptoms are present. The available data are difficult to interpret as acute pancreatitis presents relatively frequently but only occasionally in patients with COVID-19. A population-based epidemiological study to investigate the co-occurrence of pancreatitis and COVID-19 is warranted.