ABSTRACT
Systemic lupus erythematosus is a chronic autoimmune disease with a wide variety of clinical presentations induced by different immunocomplexes and autoantibodies. Antiphospholipid antibody syndrome (APLAS) is a life-threatening clinical condition characterized by venous and arterial thromboses or pregnancy morbidity in the presence of persistent moderate/high levels of antiphospholipid antibodies. Aortic dissection is rarely associated with APLAS and always requires prompt diagnosis and early treatment. We report a rare case with a striking presentation. The patient developed multi-organ failure due to lethal aortic dissection and the obstruction of abdominal and thoracic branch vessels.
LEARNING POINTS
- Aortic dissection is a rare lethal clinical condition that always requires prompt diagnosis and early treatment.
- Signs of multi-organ ischaemia due to obstruction of abdominal and thoracic branch vessels should be kept in mind by clinicians.
- Venous thrombosis and medial wall necrosis in the aorta may be underlying complex pathophysiological mechanisms in patients with antiphospholipid antibody syndrome.
KEYWORDS
Aortic dissection, antiphospholipid antibody syndrome
CASE DESCRIPTION
A 39-year-old man had presented with generalized tonic–clonic seizure 2 years previously. Cranial magnetic resonance imaging revealed a scattered subcortical ischaemic area at the level of the centrum semiovale. Antinuclear antibody (ANA) was detected homogeneously positive at 1/1250 titre, anti-dsDNA was positive, and high titre anticardiolipin IgG and lupus anticoagulant were positive on repeat measurements, revealing systemic lupus erythematosus and antiphospholipid antibody syndrome. The patient was treated with hydroxychloroquine, warfarin, mid-dose steroid, mycophenolate mofetil and carbamazepine and went into remission.
The patient was recently evaluated in the emergency room with severe, sudden onset chest and abdominal pain.
On physical examination, he had a fever of 36.8°C, pulse rate of 102 per minute, blood pressure of 90/60 mmHg, and was cooperative. Epigastric tenderness was detected and no breathing sounds could be heard the lower zone of either lung. Radial and brachial pulses were weak in the left lower extremity.
METHODS AND PROCEDURES
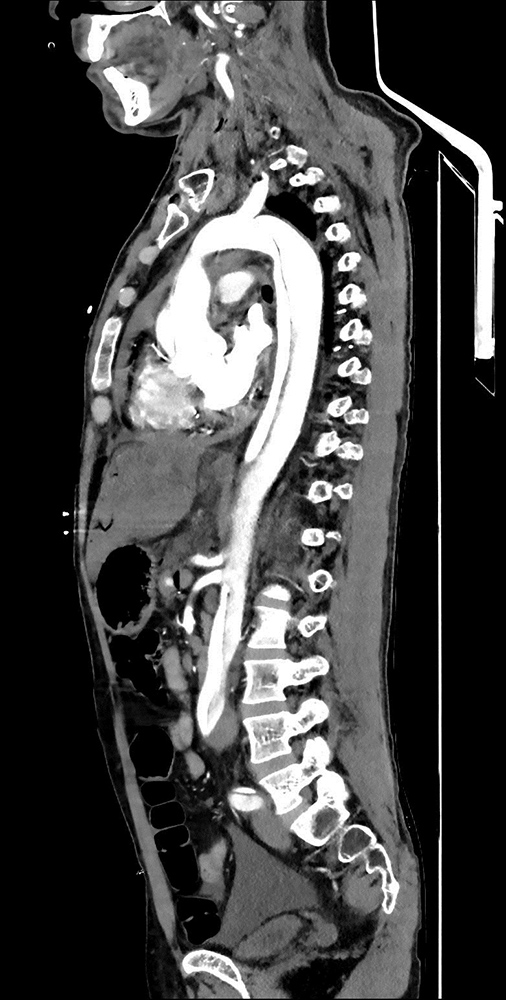
Laboratory data revealed white blood cell count 26,920, haemoglobin 17.2 g/dl, platelets 178,000, creatinine 3.5 mg/dl, aspartate aminotransferase 240 U/l, alanine aminotransferase 350 U/l, lactate dehydrogenase 940 U/l, troponin I 1040, C-reactive protein 30 mg/dl, erythrocyte sedimentation rate 45 mm/h and INR 6.5. Electrocardiography showed ST-segment elevation and T wave negativity in all leads. Pleural effusion up to the bilateral middle zones of the lungs, a 15 mm pericardial haemorrhagic effusion and a 5 cm aneurysm in the ascending aorta were revealed. Computed tomography (CT) angiographic imaging demonstrated air densities in the portal, splenic and mesenteric vessel structures, and a type 1 dissection flap along the entire thoracoabdominal aorta starting from the aortic root and extending to the left main femoral artery level (Fig. 1). After diagnosis of aortic dissection based on clinical presentation and imaging, the patient urgently received supportive treatment and close vital monitoring but died during surgery.
Figure 1. Computed tomography angiographic imaging: type 1 dissection flap along the entire thoracoabdominal aorta starting from the aortic root and extending to the left main femoral artery level
DISCUSSION
The patient was interesting in terms of admission presentation. Aortic dissection was suspected because of the anamnesis (sudden onset chest and abdomen pain) and physical examination findings (hypotension, epigastric tenderness, weak pulse). The laboratory data revealed signs of liver dysfunction, acute kidney failure and cardiac ischaemia. Although acute coronary syndrome was initially suspected, the definite diagnosis (aortic dissection) was verified by CT angiography.
Case series of APLAS patients with carotid artery dissection and coronary artery dissection have been reported [1, 2]. Our literature search revealed our case is the fourth APLAS patient to be diagnosed with aortic dissection. Sato et al. reported the first case, a 61-year-old woman with systemic lupus erythematosus (SLE) and APLAS who developed ruptured abdominal aortic dissection [3]. The second patient, a 44-year-old woman diagnosed with SLE and APLAS, also developed type A aortic dissection and was treated with ascending aorta replacement with open surgical repair [4]. Tatsvoka et al. described a 42-year-old woman diagnosed previously with primary APLAS who presented with coma and cerebellar haemorrhage. She developed type B aortic dissection triggered by acute hypertension following contrast-induced nephropathy and APLAS nephropathy [5].
Venous thrombosis and medial necrosis in the wall of the aorta have been confirmed in pathological case reports of patients with aortic dissection and APLAS. Thrombosis of the vasa vasorum induced by antiphospholipid antibodies causes vascular wall fragility and impairment of vasa vasorum blood flow leading to aortic wall necrosis [6]. The antibodies also cause direct endothelial injury and consequently accelerate the atherosclerotic process [7]. An autopsy was not performed in our patient due to his relatives’ refusal.
Old age, male sex, hypertension, disease duration of >3 years, and prolonged steroid treatment have been reported as additional risk factors for the development of aortic dissection in SLE patients [8]. Steroid inhibition of tissue repair, oxidative stress, arterial stiffness and endothelium dysfunction lead to aortic dissection, thus predisposing SLE patients to premature atherosclerosis [9]. Aortitis and the chronic inflammatory effect of SLE are also associated with aortic dissection [10]. Ultimately, an intimal tear occurs via a trigger factor in the presence of atherosclerosis and inflammation and high-flow leakage of blood into the vessel layers occurs. Our patient had no risk factors for aortic dissection, such as hypertension, dyslipidaemia, smoking, older age, longer disease duration or longer steroid use. SLE and secondary APLAS may have been be the only causative factors for aortic dissection in this patient.
CONCLUSION
We hope that this rare co-existence of APLAS, SLE and aortic dissection will raise clinician awareness of the aetiopathogenesis and presentation of this condition. The striking presentation of signs of multi-organ ischaemia due to obstruction of abdominal and thoracic branch vessels should be kept in mind by clinicians in patients with aortic dissection.