ABSTRACT
Secukinumab is an inhibitor of interleukin IL-17A, and is mainly used in the treatment of psoriasis, psoriatic arthritis and ankylosing spondylitis. Although rarely, this drug may induce paradoxical reactions, such as cutaneous vasculitis. Worldwide, only four previous cases of vasculitis induced by secukinumab have been reported. We herein report the first case described in Brazil of cutaneous vasculitis due to secukinumab in a patient with peripheral spondyloarthritis who demonstrated good resolution after withdrawal of the drug and initiation of etanercept. Clinicians should be aware of this rare but potentially serious adverse effect of secukinumab.
LEARNING POINTS
- Treatment with biologics can cause vasculitis.
- The vasculitis may be independent of class of the biologic.
- Only five cases of vasculitis induced by secukinumab have been reported.
KEYWORDS
Vasculitis, secukinumab, biological therapy
INTRODUCTION
Spondyloarthritis (SpA) is a group of inflammatory musculoskeletal disorders that share clinical characteristics, genetic susceptibility and pathophysiological mechanisms. The condition can be classified as either axial spondyloarthritis, affecting the spine and sacroiliac joints, or peripheral spondyloarthritis, where arthritis, enthesitis and/or dactylitis are the prominent clinical manifestations. Several biological treatments have emerged to control this condition, in particular those involving anti-TNF-alpha agents and, more recently, IL-12/IL-23 and IL-17 inhibitors [1].
IL-17A, which is produced by Th17 cells, mast cells and leukocytes, is a key factor in the pathogenesis of SpA, and is responsible for inflammation, enthesitis and structural damage [2]. Secukinumab is an inhibitor of IL-17A and has shown efficacy in the treatment of psoriasis, psoriatic arthritis and ankylosing spondylitis. Mens et al. also demonstrated the benefit of secukinumab in other peripheral SpA not associated with psoriasis, showing improvement in clinical disease activity parameters and the downmodulation of synovial tissue inflammation [3].
Despite the benefits of biological therapy, side effects, such as infections, infusion reactions, hypersensitivity and the development of lymphomas have been described. Curiously, there are also reports of ‘paradoxical’ reactions, which are characterized by the appearance or exacerbation of a pathological condition usually treatable by that medication.
Herein, we report the fifth case described in the world literature and the first one in Brazil of cutaneous vasculitis induced by secukinumab.
CASE PRESENTATION
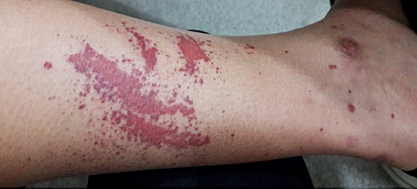
A 39-year-old female patient was diagnosed with reactive arthritis at age 13 years when she presented with diarrhoea, conjunctivitis and arthritis in her right knee and left ankle, without axial involvement. On that occasion, she was prescribed NSAIDs, corticosteroids, sulfasalazine and methotrexate. Due to the refractoriness of the condition, infliximab 5 mg/kg was added to her medication regimen, with an excellent response. However, 4 years ago, after 5 years of infliximab use, she presented with painful erythematous papules on her legs and feet (Fig. 1 and 2) that evolved into ulcers, which biopsy revealed to be leukocytoclastic vasculitis with IgA deposit by immunofluorescence. On physical examination, she was found to be well-nourished and afebrile, with a blood pressure 110/70 mmHg. Excluding the above-mentioned skin lesions, the physical examination was unremarkable.
Figure 1. Erythematous papules on the right foot suggesting cutaneous
vasculitis. Figure 2. Medial view of the left leg demonstrating a purpuric rash
Diagnostic work-up revealed a normal blood count, liver and renal profile, ESR and CRP. Complement levels were normal and viral serologies, such as anti-HCV, anti-HIV and HBsAg, were negative. Antinuclear antibody (ANA), anti-DNA, anti-Sm, anti-SSA, anticardiolipin and lupus anticoagulant were all negative and serum IgA was normal. However, C-ANCA was positive at 1/20, and HLA-B27 was also detected. Magnetic resonance imaging of the sacroiliac joints was normal.
Infliximab was withdrawn, and prednisone 1 mg/kg and then azathioprine were administered to the patient, who demonstrated gradual improvement. However, after the suspension of these medications, she again presented with peripheral inflammatory arthritis and anti-IL-17 (secukinumab) 150 mg was prescribed. Nevertheless, after 2 months, she experienced a relapse of the vasculitic lesions on her lower limbs, necessitating discontinuation of the medication. Prednisone (1 mg/kg) was re-administered in order to improve the skin disorder. Subsequently, etanercept (50 mg sc weekly) was prescribed, which relieved articular symptoms without recurrence of skin lesions. After 3 years of continuous use of this medication, the patient is doing well, with no joint or skin symptoms.
DISCUSSION
The benefits of biological therapy have been well recognized, although some adverse effects have been reported with this type of medication. One intriguing side effect is the development of ‘paradoxical’ reactions. We herein report the first case described in Brazil of cutaneous vasculitis due to secukinumab in a patient with peripheral spondyloarthritis who demonstrated good resolution after withdrawal of the drug.
This type of complication was first described with the use of anti-TNF-alpha agents, and the most frequent cutaneous paradoxical reaction is the worsening or new onset of psoriasis [4]. This reaction is not organ specific and can lead to a wide variety of complications besides psoriasis, such as suppurative hidradenitis, ulcerative colitis, uveitis, gangrenous pyoderma, small vessel cutaneous vasculitis and systemic vasculitis with renal and peripheral nervous system involvement, and medium and large vessels vasculitis [5].
By definition, paradoxical reactions are clinical manifestations which appear during the use of biological molecules classically indicated for the treatment of those conditions, for instance, paradoxical psoriasis induced by anti-TNF-alpha, uveitis in ankylosing spondylitis treated with anti-TNF-alpha, or gangrenous pyoderma in the treatment of Crohn's disease [5–7]. A paradoxical ‘borderline’ reaction is characterized by the emergence of immune-mediated conditions in which the effectiveness of the biological agent has not been widely confirmed, like the emergence of sarcoidosis and alopecia areata during therapy with anti-TNF-alpha or the occurrence of vitiligo and vasculitis during treatment with anti-TNF-alpha or anti-IL-17 as described in the present study. Sometimes it is difficult to differentiate between a loss of drug efficacy and a paradoxical adverse event [5, 7]. The pathophysiology of the paradoxical reaction is believed to involve multiple immunological pathways, leading to a cytokine imbalance [6].
With the increasing use of anti-IL-17 agents, some reports of paradoxical reactions have also been published. These include psoriasis, suppurative hidradenitis, cutaneous and intestinal vasculitis, pyoderma gangrenosum, Behçet's disease, and cutaneous and intestinal vasculitis [6, 8–11]. Drug-induced small vessel vasculitis is mediated by immune complex deposition. These medications can induce the production of antigens which lead to the formation of antibodies; these immune complexes are deposited within vessels, resulting in complement activation and the inflammatory process [12].
Anti-TNF-alpha inhibitors are the most common biological agents capable of inducing vasculitis, with leukocytoclastic vasculitis being the most common finding. Palpable purpura is the most frequent manifestation, but nodules and ulcerated lesions have also been described. Additionally, systemic vasculitis has been reported, predominantly involving the renal and peripheral nervous systems. Drug withdrawal results in improvement in about 90% of cases [7, 13]. Relapse of the vasculitis can occur in 67% of cases when there is re-exposure to the same TNF inhibitor; this figure drops significantly to 33% when another TNF inhibitor is tested [14]. In cases where a biological medication is necessary for control of the underlying disease, there is no agreement regarding the optimal medication protocol, that is, whether switch to one with a different mechanism of action or use another agent within the same class. In the present study, the patient experienced a recurrence of the vasculitis when a different biological class (anti-IL-17) was administered, but, curiously, retreatment with a second anti-TNF agent was uneventful [4].
Vasculitis secondary to secukinumab has only been described in four previous patients. Leukocytoclastic vasculitis induced by secukinumab was described in a 28-year-old female patient after 8 months of using the drug to treat psoriasis, with improvement of the lesions after discontinuation and administration of prednisone and cyclosporine [15]. Chelli et al. reported a patient with psoriatic arthritis who had been using secukinumab for 1 month and presented with psoriasiform lesions on her vulva, palpable purpura on her lower limbs, and intestinal vasculitis. This complication improved after the drug was withdrawn and 1 mg/kg/day prednisone and colchicine were administered [8]. The remaining two cases were diagnosed with Behçet's disease secondary to anti-IL-17 therapy for psoriasis. Drug withdrawal and the administration of corticosteroids resolved the problem [10].
Despite being a rare manifestation, the early recognition of vasculitis as an adverse event of biological therapy is very important. New studies are necessary to clarify the immunological mechanisms, genetic factors, and possible triggers that can induce such autoimmune manifestations.